A brief synopsis of phycological studies in Uruguay1
Una sinopsis breve de los estudios ficológicos en Uruguay
Sylvia Bonilla
Sección Limnología, Facultad de Ciencias, Universidad de la República, Iguá 4225, 11400, Montevideo, Uruguay
Corresponding author: email:sbon@fcien.edu.uy
1 In memory of the Uruguayan taxonomist María del Carmen Pérez (1959-2022).
1 En memoria de la taxónoma uruguaya María del Carmen Pérez (1959-2022).
Bonilla, S. 2022. A brief synopsis of phycological studies in Uruguay. Cymbella 8(1): 5-13. https://cymbella.mx
Abstract
In this article, I summarize the development of the phycology in Uruguay since the beginning of the 19th Century. Uruguayan phycological research has been dominated by ecological, eco-physiological and taxonomical studies of freshwater phytoplankton, influenced by the development of limnology, with few studies completed on marine macroalgae. Other areas such as biotechnology, evolution and the biology of algae and cyanobacteria are still largely unexplored. I pay homage to María del Carmen Pérez (1959-2022), who was the most important Uruguayan phytoplankton taxonomist ever, and a kind, enthusiastic and hard-working person with a distinctive passion for phytoplankton taxonomy.
Keywords: blooms, history, macroalgae, phytoplankton, taxonomy.Resumen
En este artículo realizo un repaso del desarrollo de la ficología en Uruguay desde comienzos del siglo 19. La investigación en ficología en Uruguay ha estado centrada en la ecología, la ecofisiología y la taxonomía de fitoplancton de agua dulce, influido por el desarrollo de la limnología. Existen muy pocos trabajos referidos a macroalgas. Otras áreas como la biotecnología, la evolución y la biología de las algas y cianobacterias, permanecen mayormente inexploradas. Rindo homenaje a María del Carmen Pérez (1959–2022), quien fuera la taxónoma de fitoplancton más relevante de Uruguay, una persona amigable, entusiasta y trabajadora incansable, con una gran pasión por el fitoplancton.
Palabras clave: fitoplancton, floraciones, historia, macroalgas, taxonomía.Located at the southern limit of the subtropical climate zone, Uruguay is one of the smallest and least populated countries in South America (between 30° and 35° S, and 53º and 58° W). The small territory (176,200 km2) is crossed by a rich freshwater network of rivers, streams, and shallow lakes. Uruguay’s coastline on the Río de la Plata estuary and the Atlantic Ocean extends for more than 600 km. Despite the numerous aquatic ecosystems, its phycological history is relatively brief and encompasses the development of other biological and environmental disciplines. In this work I refer to phycology in its broadest sense, including the taxonomy, biology, physiology or ecology of macro and microalgae (as well as cyanobacteria).
The first algal studies were carried out in the 19th century by naturalists that listed species of algae found in freshwaters and marine coasts. José Arechavaleta (1838–1912) was an active scientist and promoter of the natural sciences and was probably the first systematist collecting and identifying flora (plants and algae) in the country (Arechavaleta 1883, 1884). Florentino Felippone (1852-1939) was another notably prolific and curious naturalist; he was responsible for the first samples identified from the country, having developed a list of 22 species of green and red algae including a new species that was named after him (Callithamnion felipponei Howe 1931). Around that time, the cyanobacterium Spirulina jenneri (Gomont) Geitler was first described from samples collected in a pond near Montevideo (Gomont 1892). Taxonomic studies, or species lists, were reported in the early 20th Century, mostly with reference to freshwater and marine diatoms and green algae (Allen & Herter 1934; Carbonell & Pascual 1925; Frenguelli 1933a, b; Muller-Melchers 1953; Santibañez 1939). The country’s public university, the Universidad de la República, was created in 1849 with few program options. Later, in 1945, the Facultad de Humanidades y Ciencias (Faculty of human and sciences studies) was incorporated and it boosted the development of natural sciences, although areas such as botany, limnology and phycology were virtually absent.
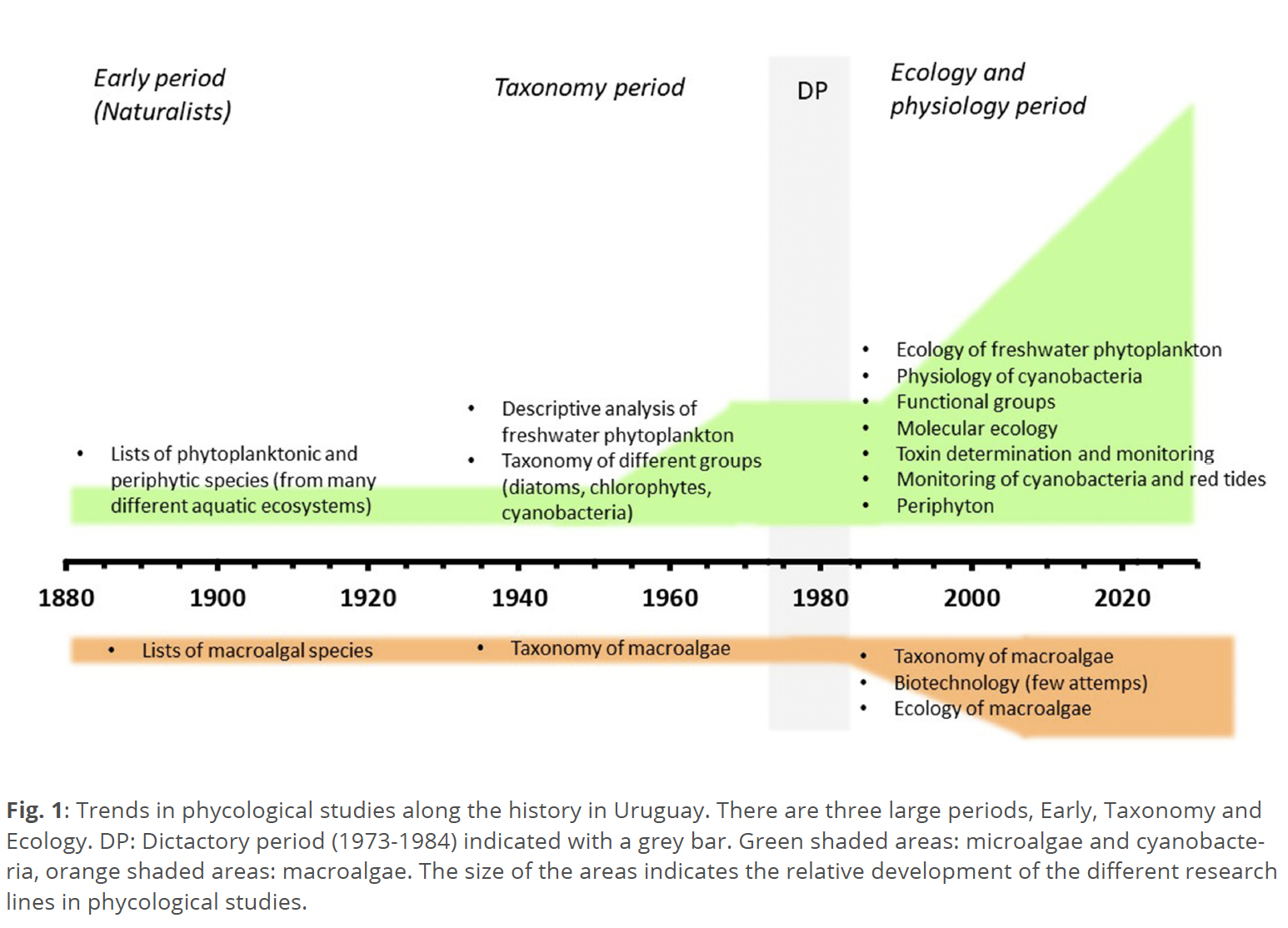
The main areas of phycological research developed in Uruguay followed the main trends of research carried out in Latin America. The study of the taxonomy of microalgae and cyanobacteria have a long tradition in Latin America, strongly influenced by the European schools and focused on descriptive studies or the compilation of species lists (Irfanullah 2006). In the first half of the 20th Century, Uruguay was part of that trend, although with a slower development in comparison with their neighbors (Argentina and Brazil). Until late 1980s, most publications from Uruguayan waterbodies were species lists, mainly of microalgae and cyanobacteria (Coll 1979) (Fig. 1).
The development of limnology in Latin America, initially centered in the study of lakes and communities, was also influenced by the European schools (Elster 1974; Tundisi & Matsumura-Tundisi 2011). Freshwater phytoplankton ecology studies increased after ~1930 in Brazil and somewhat later in other countries (~1950s in Colombia and Argentina, ~1960 in Ecuador, and ~1984 in Uruguay) (de Buen 1950; Conde & Sommaruga 1999; López & Mariazzi 1994; Roldán 2020; Steinitz-Kannan et al. 2020). The Uruguayan military dictatorship (1973–1984) had a strong impact on slowing scientific research, dismantling existing research groups and leaving the country in great academic isolation (Markarian 2015). The creation of the Limnology Division at the Universidad de la República in 1984, coupled with the increased awareness of environmental issues and the appreciation of water as a resource, favored the development of limnological studies, including phytoplankton (Conde & Sommaruga 1999) (Fig. 1). Cyanobacterial toxic blooms in freshwaters of the country have been increasing for decades in response to eutrophication, affecting the major watersheds and restricting the use of the water (Aubriot et al. 2020; Bonilla et al. 2015; Conde et al. 2002; De León & Yunes 2001; Goyenola et al. 2021). This phenomenon had gained great attention in limnological research, particularly after the 1990s. Studies related with microalgae and cyanobacteria in freshwaters included taxonomy and ecology (i.e.: Bonilla 2009; Bonilla et al. 2005, 2006; De León & Chalar 2003; Ferrari 2020; González-Madina et al. 2017; Haakonsson et al. 2017; Kruk et al. 2009; Pacheco et al. 2021), ecology with emphasis in functional groups (i.e.: Kruk et al. 2002; Pacheco et al. 2010; Segura et al. 2013), ecophysiology of cyanobacteria (i.e.: Aguilera et al. 2017, 2019; Amaral et al. 2014; Aubriot 2019; Aubriot & Bonilla 2018; Aubriot et al. 2011; Beamud et al. 2016; Bonilla 2012; Fabre et al. 2017), molecular studies of cyanobacteria (i.e.: Beamud et al. 2016; Martínez de la Escalera et al. 2017, 2019; Piccini et al. 2011; Rigamonti et al. 2019; Vico et al. 2016) and phylogenetic studies of cyanobacteria (Vico et al. 2020). Despite the vast network of rivers and the presence of shallow lakes, favorable environments for the periphyton, studies on the taxonomy and structure of the periphyton are still scarce (Bonilla 1997; Pacheco 2016). A special mention deserves María del Carmen Pérez, the most prominent Uruguayan taxonomist, to whom I pay homage in the next section.
The first study of coastal marine phytoplankton was carried out by an expedition during the early 1930s (Hentschel 1932, in Ferrari & Vidal 2006). Early studies about the ecology of coastal phytoplankton described the distribution of species in relation to main environmental factors (Bayssé et al. 1986, 1989; Ferrando 1962). In February 1980, a massive red tide resulted in an event of acute human neurotoxin intoxication due to the consumption of contaminated mussels (Galasso et al. 1980). This event trigged the implementation of the national red tide monitoring program, including the determination of dinoflagellate species and toxin detection (Méndez 2006). Red tides have been increasing in magnitude and duration during the last several decades along the marine coasts of the country, where toxic Dinophysis cf. acuminata Claparède & Lachmann is becoming more frequent, probably associated to eutrophication and climate change effects (Martínez et al. 2017). Most of the common phytoplankton blooms along the coast of the country are of cyanobacteria (Microcystis aeruginosa (Kützing) Kützing complexand Dolichospermum spp., dinoflagellates (Ceratium hirundinella (O.F. Müller) Dujardin, and the marine Alexandrium tamarense (Lebour) Balech and Gymnodinium catenatum Graham) and diatoms (Aulacoseira sp., Skeletonema costatum (Greville) Cleve, Leptocylindrus spp., Chaetoceros spp., Rhizosolenia spp., among others) (Bonilla 2009; Ferrari & Méndez 2000; Ferrari & Vidal 2006; Haakonsson et al. 2017; Kruk et al. 2019; Martínez et al. 2017; Méndez 2006; Pacheco et al. 2021). Research about the physiology of benthic cyanobacteria growing in rice fields (Irisarri et al. 2001, 2007; Pérez et al. 2012, 2020) is probably the only research line related with biotechnology. A few other studies were focused on the lipidic content of microalgae (Pagano 1993; Pagano et al. 1998).
Macroalgae have been largely overlooked or neglected in scientific studies in Uruguay, and the knowledge of the macroalgal flora is still limited (Coll & Oliveira 1999) (Fig. 1). Most of the 670 km of Uruguayan coastline is sandy, with few rocky habitats for marine macroalgae. The large influence of the estuary Río de la Plata, one of the largest in the world, implies a highly dynamic environment with frequent and large changes in turbidity and salinity, limiting the number of algal species that tolerate those conditions. In a survey conducted by the phycologist Javier Coll, compiling information along the Uruguayan coast, macroalgal species were mainly characteristic of subtropical or warm temperate regions (45% and 38% of the total, respectively) or were cosmopolitan. Coll listed 53 species of red algae (where the most frequent and common species were Cryptopleura ramose (Hudson) Newton, Chondria atropurpurea Harvey, Corallina officinalis Linnaeus,
Gymnogongrus griffithsiae (Turner) Martius, Hypnea musciformis (Wulfen) Lamouroux and Jania rubens (Linnaeus) Lamouroux) and 26 species of green algae (from the genera Bryopsis, Chaetomorpha, Cladophora, Codium, Enteromorpha, Ulva and Rhizoclonium) (Coll & Oliveira 1999). The brackish conditions that dominated large areas of the coastline were pointed out as the main factor for the presence of only 8 species of brown algae, where Scytosiphon lomentaria (Lyngbye) Link was the most frequent and abundant (Coll & Oliveira 1999). Higher temperatures during summer play a role in explaining an increase in the richness and biomass of subtropical species on rocky marine shores (González-Etchebehere et al. 2017). A recent study that focused on the ecology of macroalgae proposed morpho-functional groups as an innovative approach to study these organisms (Vélez-Rubio, et al., 2021). There are only few attempts evaluating biotechnological uses of agar-producing marine macroalgae (Porzekanski et al. 1965).
María del Carmen Pérez (1959–2022)
María del Carmen Pérez was an enthusiastic and highly dedicated phytoplankton taxonomist. Starting as an autodidact, she quickly became in an expert, and developed into the best and most experienced taxonomist in Uruguay ever. Being a pioneer, she started working on taxonomy of phytoplankton with no guidance and few resources, motivated only by her interest and passion. María del Carmen started her bachelor’s degree in biology in 1984 at the Universidad de la República in Montevideo. Several years later she joined the Botany Department as an honorary collaborator. Although the department was focused on mycology, María del Carmen had her very first opportunity to observe hundreds of water samples under a microscope. At that time there were no experts in academia working in taxonomy or ecology of phytoplankton. The university was still recovering from the repression during the military dictatorship, which implied the absence of specialized literature and good facilities for research. However, those harsh conditions never discouraged her; on the contrary, she devoted herself with great passion to observing fresh samples that she herself collected. She worked on the weekends when the lab was available. During that period, María del Carmen became amazed by the diversity of forms and organisms that passed in front of her eyes, and her burgeoning excitement for taxonomy increased exponentially. In the early 1990s she took specialized courses and did internships about taxonomy and biology of freshwater microalgae in Argentina and Brazil. Her enthusiasm and outgoing personality ensured that she had no barriers to making friends and establishing strong academic relationships with colleagues and experts from other countries. Later, she worked in tight collaboration with many taxonomists, among others, Nora Maidana (Argentina), Augusto Comas (Cuba) and Célia Leite Sant’Anna (Brazil). After finishing her studies at the Universidad de la República (Bachelor in Biological Sciences, 1994) she moved to Spain, where she continued her postgraduate studies (DEA: advanced studies diploma, Universidad Politécnica de Valencia, 2010; and doctoral studies at the same university). She visited the laboratory of Professor Jiri Komárek (Czech Republic) to work in cyanobacteria, with whom she established a long-lasting academic cooperation. María del Carmen worked as a professional in phytoplankton taxonomy in different institutions along her prolific and active career, in Uruguay (the national fisheries resources: DINARA, the technological laboratory: LATU, the national water facility: OSE and the Universidad de la República) and Spain (Universitat Politècnica de València, Conselleria de Medi Ambient, Universidad de Girona). She analyzed phytoplankton samples with optical and electronic microscopes (Fig. 2), worked actively in monitoring programs of red tides and water quality (DINARA and LATU), monitoring of phytoplankton and cyanobacteria in water sources (OSE) and in many research projects related with phytoplankton ecology (Facultad de Ciencias, Universidad de la República) (Fig. 2). Wherever she has worked, she has stood out for her hard, meticulous, and rigorous work. She became an academic reference in taxonomy of phytoplankton, frequently consulted by Uruguayan colleagues.
María del Carmen loved teaching young researchers in practical courses. She dedicated many hours to the training of technicians and postgraduate students who were beginning their studies in phytoplankton taxonomy (Fig. 2). Her contagious enthusiasm spread easily to the students, and she always provided relevant information for species identification, details not found in books and that are only learned from experience.
María del Carmen significantly contributed to the knowledge of the microalgae flora of Uruguay. She characterized for the first time the phytoplankton communities of important freshwaters such as the Río Negro (Conforti & Pérez 2000; Pérez 2002; Pérez et al. 1999b), the large Merín Lagoon (Pérez & Odebrecht 2005; Sophia & Pérez 2010), the Uruguay River (Ferrari et al. 2011) and coastal lagoons (Bonilla et al. 2005). She significantly contributed to the knowledge of the phycological flora of Uruguay, including the discussion of the type material of Nodularia spumigena Mertens ex Bornet & Flahault from a coastal lagoon (Pérez et al. 1999a) and the discovery of a new species of cyanobacteria Dolichospermum uruguayense Kozlíková-Zapomělová & al. (Kozlíková-Zapomêlová et al. 2016). She greatly contributed to the taxonomy and ecology of brackish and freshwater phytoplankton of Spanish ecosystems (Carrillo et al. 2008; Falco et al. 2006; Pérez & Carrillo 2005; Pérez et al. 2009, 2010; Tornés et al. 2014); also she found two new chlorophyte species: Pediastrum willei Comas et al. and Lobocystis fottiana Comas et al. (Comas & Pérez 2002; Comas Gonzalez et al. 2006). Beyond her technical contributions, her biggest legacy may be her enthusiasm and generosity in sharing her knowledge. She always maintained her openness and her ability to be surprised by new organisms, and by samples with rare species, and she was able to pass this enthusiasm to the people around her.
Future challenges
Unfortunately, Uruguay is one of the countries with the lowest investment in science and technology of Latin America, which greatly limits the development of new lines of research (Ciocca & Delgado 2017). In this context, the most important challenges for phycology in Uruguay are related to the development of new areas, especially those related to basic studies (physiology or evolution) to generate the bases that promote applied areas such as biotechnology. More basic studies of the molecular biology of algae (macro and micro), and more studies related to the periphyton (biology, physiology, and ecology) are also needed.
Acknowledgements
I thank the editors of Cymbella for their guidance and assistance, Dermot Antoniades for English assistance, and Graciela Ferrari, Agustín Manjón and Vicent Benedito Durá who kindly gave me the permission to use the photographs of María del Carmen Pérez.
REFERENCIAS
Aguilera, A., L. Aubriot, R. O. Echenique, J. L. Donadelli & G. L. Salerno. 2019. Raphidiopsis mediterranea (Nostocales) exhibits a flexible growth strategy under light and nutrient fluctuations in contrast to Planktothrix agardhii (Oscillatoriales). Hydrobiologia 839: 145-157. https://doi.org/10.1007/s10750-019-04002-5.
Aguilera, A., L. Aubriot, R. O. Echenique, G. L. Salerno, B. M. Brena, M. Pírez & S. Bonilla. 2017. Synergistic effects of nutrients and light favor Nostocales over non-heterocystous cyanobacteria. Hydrobiologia 794. https://doi.org/10.1007/s10750-017-3099-1
Allen, G. O. & W. G. Herter. 1934. Charales uruguayensis. Revista Sudamericana de Botánica 1: 87-91.
Amaral, V., S. Bonilla & L. Aubriot. 2014. Growth optimization of the invasive cyanobacterium Cylindrospermopsis raciborskii in response to phosphate fluctuations. European Journal of Phycology 49: 134-141. https://doi.org/10.1080/09670262.2014.897760
Arechavaleta, J. 1883. Los Vaucheria Montevideanos. Anales del Ateneo del Uruguay 4: 18-28.
Arechavaleta, J. 1884. Lecciones de botánica médica. Revista de la Sociedad Universitaria 1: 305-320.
Aubriot, L. 2019. Nitrogen availability facilitates phosphorus acquisition by bloom-forming cyanobacteria. FEMS Microbiology Ecology 95: 1-10. https://doi.org/10.1093/femsec/fiy229
Aubriot, L. & S. Bonilla. 2018. Regulation of phosphate uptake reveals cyanobacterial bloom resilience to shifting N: P ratios. Freshwater Biology 63: 318-329. https://doi.org/10.1111/fwb.13066
Aubriot, L., S. Bonilla & G. Falkner. 2011. Adaptive phosphate uptake behaviour of phytoplankton to environmental phosphate fluctuations. FEMS Microbiology Ecology 77: 1-16. https://doi.org/10.1111/j.1574-6941.2011.01078.x
Aubriot, L., B. Zabaleta, F. Bordet, D. Sienra, J. Risso, M. Achkar & A. Somma. 2020. Assessing the origin of a massive cyanobacterial bloom in the Río de la Plata (2019): Towards an early warning system. Water Resources 181. https://doi.org/10.1016/j.watres.2020.115944
Bayssé, C., J. C. Elgue & F. Burone. 1989. Variaciones en la distribución y relaciones interespecíficas del fitoplancton en una playa arenosa de la costa atlántica uruguaya. Publicaciones la Comisión Técnica Mixta del Frente Marítimo 5(A): 95-114.
Bayssé, C., J. C. Elgue, F. Burone & M. Parietti. 1986. Campaña de invierno 1983. II Fitoplancton. Publicaciones la Com. Técnica Mixta del Frente Marítimo 1: 218-229.
Beamud, G., P. Vico, S. Haakonsson, G. Martínez de la Escalera, C. Piccini, B. M. Brena, M. Pirez & S. Bonilla. 2016. Influence of UV-B radiation on the fitness and toxin expression of the cyanobacterium Cylindrospermopsis raciborskii. Hydrobiologia 763: 161-172. https://doi.org/10.1007/s10750-015-2370-6
Bonilla, S. 1997. Estructura y dinamica de la comunidad epifitica algal en un sistema somero mixohalino. Tesis de maestría. Universidad de la República, Montevideo.
Bonilla, S. 2009. Cianobacterias Planctónicas del Uruguay. Manual para la identificación y medidas de gestión, PHI-VII/UNESCO, Montevideo. Disponible en: https://unesdoc.unesco.org/ark:/48223/pf0000216319.locale=en
Bonilla, S., L. Aubriot, M. C. S. Soares, M. González-Piana, A. Fabre, V. L. Huszar, M. Lürling, D. Antoniades, J. Padisák & C. Kruk. 2012. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiology Ecology 79. doi:10.1111/j.1574-6941.2011.01242.x
Bonilla, S., D. Conde, L. Aubriot & M. D. C. Pérez. 2005. Influence of hydrology on phytoplankton species composition and life strategies in a subtropical coastal lagoon periodically connected with the Atlantic Ocean. Estuaries 28: 884-895. https://doi.org/10.1007/BF02696017
Bonilla, S., D. Conde, L. Aubriot, L. Rodríguez-Gallego, C. Piccini, E. Meerhoff, L. Rodríguez-Grana, D. Calliari, P. Gómez, I. Machado & A. Britos. 2006. Procesos estructuradores de las comunidades biológicas en lagunas costeras de Uruguay,. In R. Menafra, L. Rodríguez-Gallego, F. Scarabino, and D. Conde. Eds. Bases para la conservación y el manejo de la costa uruguaya. Vida Silvestre Uruguay, Montevideo, p. 611-630
Bonilla, S., S. Haakonsson, A. Somma, A. Gravier, A. Britos, L. Vidal, L. De León, B. Brena, M. Pírez, C. Piccini, G. Martínez de la Escalera, G. Chalar, M. González-Piana, M. Martigani & L. Aubriot. 2015. Cianobacterias y cianotoxinas en ecosistemas límnicos de Uruguay. INNOTEC 10: 9-22. https://doi.org/10.26461/10.01
de Buen, F. 1950. La oceanografía y la limnología en campañas y laboratorios. Revista de la Facultad de Humanidades y Ciencias 4: 221-228.
Carbonell, J. J. & A. Pascual. 1925. Una Melosira nueva pra el Río de la Plata. Physis 8: 106-107.
Carrillo, A., P. Huq, M. C. Pérez & J. M. Redondo. 2008. Spatial and temporal variation of picoplanktic cyanobacteria population in a density stratified estuary, and the introduction of a cellular gradient number. Estuarine Coastal and Shelf Science 76: 153-162. https://doi.org/10.1016/j.ecss.2007.06.015
Ciocca, D. R. & G. Delgado. 2017. The reality of scientific research in Latin America; an insider’s perspective. Cell Stress Chaperones 22: 847-852. https://doi.org/10.1007/s12192-017-0815-8
Coll, J. 1979. Catálogo de algas citadas para el Uruguay. Servicio de Oceanografía, Hidrografía y Meterología de la Armada, Oficina Regional de Ciencia y Tecnología de la Unesco para América Latina y el Caribe.
Coll, J. & E. C. Oliveira. 1999. The benthic marine algae of Uruguay. Botanica Marina 42: 129-135. https://doi.org/10.1515/BOT.1999.016
Comas, A. & M. d. C. Pérez. 2002. Chlamydophyceae (Chlorophyceae) from Merin lagoon (Brazil-Uruguay, South America) with special references to the family Botryococcaceae. Algological Studies /Archiv für Hydrobiologie. Supplement 107: 49-65. https://doi.org/10.1127/algol_stud/107/2002/49
Comas Gonzalez, A., M. C. Pérez & J. González del Río Rams. 2006. Pediastrum willei nom. et sp. nov.(Chlorophyta, Neochloridales) from the Ebro river (Spain) and its relations to P. muticum Kutz. sensu Brunnthaler 1915 pro parte. Algological Studies /Archiv für Hydrobiologie. Supplement 120: 5-13. https://doi.org/10.1127/1864-1318/2006/0120-0005
Conde, D., R. Arocena & L. Rodríguez. 2002. Recursos acuáticos sperficiales de Uruguay: Ambientes, algunas problemáticas y desafios para la gestión. Las Aguas Superficiales Continentales. Ambios 10: 5-9 y 32-33.
Conde, D. & R. Sommaruga. 1999. A review on the state of Limnology in Uruguay,. In R.G. Wetzel &B. Gopal. Eds. Limnology in Developing Countries II. International Association for Limnology – International Scientific Publications, New Delhi. pp. 1-31.
Conforti, V. & M. C. Pérez. 2000. Euglenophyceae of Negro River, Uruguay, South America. Algological Studies /Archiv für Hydrobiologie. Supplement. 97: 59-78. https://doi.org/ 10.1127/algol_stud/97/2000/59
De León, L. & G. Chalar. 2003. Abundancia y diversidad del fitoplancton en el Embalse de Salto Grande (Argentina - Uruguay). Ciclo estacional y distribución espacial. Limnetica 22: 103-113. https://doi.org/10.23818/limn.22.07
De León, L. & J. Yunes. 2001. First report of a Microcystis aeruginosa toxic bloom in La Plata River. Environmental Toxicology. 16: 110-112. DOI: 10.1002/1522-7278(2001)16:1<110::aid-tox1012>3.0.co;2-z
Elster, H.-J. 1974. History of limnology. SIL Communications, 1953-1996 20: 7-30. https://doi.org/10.1080/05384680.1974.11923880
Fabre, A., G. Lacerot, R. R. de Paiva, M. C. S. Soares, V. F. de Magalhães & S. Bonilla. 2017. South American PSP toxin-producing Cylindrospermopsis raciborskii (Cyanobacteria) decreases clearance rates of cladocerans more than copepods. Hydrobiologia 785. https://doi.org/1007/s10750-016-2903-7
Falco, S., I. Romero, M. Rodilla, J. P. Sierra, J. G. del Río, C. Mosso & M. C. Perez. 2006. Chlorophyll a and phytoplankton maximum at the halocline of Ebro River Estuary. Journal of Coastal Research S39: 526-530.
Ferrando, H. J. 1962. Frecuencia estacional del microplancton costero de Montevideo durante el año 1959. Ministerio de Industria y Trabajo. Servicio Oceanografico y de Pesca 1: 1-28.
Ferrari, G. 2020. Water flow and temperature as main factors that regulate phytoplankton and cyanobacterial blooms in a large subtropical river. INNOTEC 20: 30-66. https://doi.org/10.12461/20.07
Ferrari, G. & S. Méndez. 2000. Report of phytoplankton species producing coastal water discoloration in Uruguay. Iheringia, Série Botânica 54: 3-18.
Ferrari, G., M. d. C. Pérez, M. Dabezies, D. Míguez & C. Saizar. 2011. Planktic Cyanobacteria in the Lower Uruguay River, South America. Fottea 11: 225–234. https://doi.org/10.5507/fot.2011.021
Ferrari, G. & L. Vidal. 2006. Fitoplancton de la zona costera uruguaya: Rio de la Plata y Océano Atlántico. In: R. Menafra, L. Rodríguez-Gallego, F. Scarabino, and D. Conde. Eds. Bases para la Conservación y el Manejo de la Costa Uruguaya. Vida Silvestre Uruguay, Montevideo, pp. 45-56.
Frenguelli, J. 1933a. Diatomeas de Montevideo. Ostenia 1: 122-130.
Frenguelli, J. 1933b. Nitzschia (Nitzshiella) ventricosa Palmer, merítica en el litoral del Atlántico del Uruguay. Notas del Museo de La Plata, Sección Botánica 10: 137-142.
Galasso, A., H. Grela & C. Hartmann. 1980. Intoxicacion aguda por mejillones. Epidemia de 1980 en Uruguay. Revista del Servicio de Sanidad de las FFAA: 29-34.
Gomont, M. 1892. Monographie des Oscillariées. Annales des Sciences Naturalles. Septieme Serie. Botanique.Tome Quinzieme. G. Masson, Editeur, Paris, 15: 1-367.
González-Etchebehere, L., C. Kruk, F. Scarabino, M. Laporta, M. Zabaleta, L. González & G, Vélez-Rubio. 2017. Comunidades de macroalgas en puntas rocosas de la costa de Rocha, Uruguay. INNOTEC 14. https://:doi.org/10.26461/14.07
González-Madina, L., J. P. Pacheco, N. Mazzeo, P. Levrini, J. M. Clemente, J. J. Lagomarsino & C. Fosalba. 2017. Factores ambientales controladores del fitoplancton con énfasis en las cianobacterias potencialmente tóxicas en un lago somero utilizado como fuente de agua para potabilización: Laguna del Sauce, Maldonado, Uruguay. INNOTEC 13: 26-35. https://doi.org/10.2646/13.03
Goyenola, G., C. Kruk, N. Mazzeo, A. Nario, C. Perdomo, C. Piccini & M. Meerhoff. 2021. Producción, nutrientes, eutrofización y cianobacterias en Uruguay: armando el rompecabezas. INNOTEC 22: e558. https://doi.org/10.26461/22.02
Haakonsson, S., L. Rodríguez-Gallego, A. Somma & S. Bonilla. 2017. Temperature and precipitation shape the distribution of harmful cyanobacteria in subtropical lotic and lentic ecosystems. Science of the Total Environment 609: 1132-1139. http://doi.org/10.1016/j.scitotenv.2017.07.067.
Howe, M. A. 1930. Notes on the Algae of Uruguay. Bulletin of the Torrey Botanical Club 57: 605-610. https://doi.org/10.2307/2480570
Irfanullah, H. M. 2006. Algal taxonomy in limnology: An example of the declining trend of taxonomic studies? Hydrobiologia 559: 1–9. https://doi.org/10.1007/s10750-005-9202-z
Irisarri, P., S. Gonnet, E. Deambrosi & J. Monza. 2007. Cyanobacterial inoculation and nitrogen fertilization in rice. World Journal of Microbiology and Biotechnology 23: 237-242. https://doi.org/10.1007/s11274-006-9219-0
Irisarri, P., S. Gonnet & J. Monza. 2001. Cyanobacteria in Uruguayan rice fields: Diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. Journal of Biotechnology 91: 95-103. https://doi.org/10.1016/s0168-1656(01)00334-0
Kozlíková-Zapomêlová, E., G. Ferrari & M. d. C. Pérez. 2016. Dolichospermum uruguayense sp. nov., a planktic nostocacean cyanobacterium from the Lower Uruguay River, South America. Fottea 16: 189-200. https://doi.org:10.5507/fot.2016.009
Kruk, C., A. Martínez, G. Martínez de la Escalera, R. Trinchin, G. Manta, A. M. Segura, C. Piccini, B. Brena, G. Fabiano, M. Pirez, L. Gabito, I. Alcántara & B. Yannicelli. 2019. Floración excepcional de cianobacterias tóxicas en la costa de Uruguay, verano 2019. INNOTEC 18: 36-68. https://doi.org/10.26461/18.06
Kruk, C., N. Mazzeo, G. Lacerot & C. S. Reynolds. 2002. Classification schemes for phytoplankton: A local validation of a functional approach to the analysis of species temporal replacement. Journal of Plankton Research 24: 901-912. https://doi.org/10.1093/plankt/24.9.901
Kruk, C., L. Rodríguez-Gallego, M. Meerhoff, F. Quintans, G. Lacerot, N. Mazzeo, F. Scasso, J. C. Paggi, E. T. H. M. Peeters & S. Marten. 2009. Determinants of biodiversity in subtropical shallow lakes (Atlantic coast, Uruguay). Freshwater Biology 54: 2628-2641. https://doi.org/10.1111/j.1365-2427.2009.02274.x
López, H. L. & A. A. Mariazzi. 1994. Limnology in Argentina: an historical account. Freshwater Forum 4: 169-178. Accesible en: http://sedici.unlp.edu.ar/bitstream/handle/10915/50700/Documento_completo.pdf?sequence=1&isAllowed=y
Markarian, V. 2015. La universidad intervenida. Cambios y permanencias de la educación superior uruguaya durante la última dictadura (1973-1984). Cuadernos Chilenos de Historia de la Educación 4: 121-152.
Martínez, A., S. Méndez, A. Fabre & L. Ortega. 2017. Intensificación de floraciones de dinoflagelados marinos en Uruguay. INNOTEC 13: 19-25. https://doi.org/10.26461/13.02
Martínez de la Escalera, G., C. Kruk, A. M. Segura, L. Nogueira, I. Alcántara & C. Piccini. 2017. Dynamics of toxic genotypes of Microcystis aeruginosa complex (MAC) through a wide freshwater to marine environmental gradient. Harmful Algae 62: 73-83. https://doi.org/ 10.1016/j.hal.2016.11.012
Martínez de la Escalera, G., A. Segura, C. Kruk, B. Ghattas & C. Piccini. 2019. Genotyping and functional regression trees reveals environmental preferences of toxic cyanobacteria (Microcystis aeruginosa complex) along a wide spatial gradient. BioRxiv. doi:10.1101/2019.12.20.885111
Méndez, S. M. 2006. Impacto de las floraciones algales nocivas en Uruguay: orígen, dispersión, monitoreo, control y mitigación.,. In: R. Menafra, L. Rodríguez-Gallego, F. Scarabino & D. Conde. Eds. Bases para la Conservación y el Manejo de la Costa Uruguaya. Vida Silvestre Uruguay, Montevideo, pp. 57-69
Muller-Melchers, F. C. 1953. New and little known diatoms from Uruguay and the South Atlantic Coast. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo 3:1-11, 7 pls.
Pacheco, J. P. 2016. Efectos de la estructura trófica sobre el fitoplancton y perifiton en lagos someros subtropicales y templados. Tesis de Maestría. Universidad de la República, Montevideo.
Pacheco, J. P., C. Iglesias, M. Meerhoff, C. Fosalba, G. Goyenola, F. Teixeira-de Mello, S. García, M. Gelós & F. García-Rodríguez. 2010. Phytoplankton community structure in five subtropical shallow lakes with different trophic status (Uruguay): A morphology-based approach. Hydrobiologia 646: 187-197. https://doi.org/10.1007/s10750-010-0180-4
Pacheco, J. P., C. Iglesias Frizzera, G. Goyenola, F. Teixeira de-Mello, C. Fosalba, A. Baattrup-Pedersen, M. Meerhoff & E. Jeppesen. 2021. Invasion of Ceratium furcoides in subtropical lakes in Uruguay: Environmental drivers and fish kill record during its bloom. Biological Invasions 23: 3597–3612. https://doi.org/10.1007/s10530-021-02600-w
Pagano, T. 1993. Composición lipídica de microalgas autóctonas del Uruguay e influencia de las condiciones de cultivo. Tesis de Maestría. Universidad de la República. 155 pp.
Pagano, T., J. Coll & M. A. Grompone. 1998. Chlorella vulgaris: composición y cultivo de una microalga colectada en Uruguay. Ingeniería Química 14: 47-54.
Pérez, G., S. Doldán, O. Borsani & P. Irisarri. 2012. Differential response to moderate UV-B irradiation of two heterocystous cyanobacteria isolated from a temperate ricefield. Advances in Microbiology 2: 37-47. http://doi.org/10.4236/aim.2012.21006
Pérez, G., V. Cerecetto & P. Irisarri. 2020. Potential cyanobacterial inoculants for rice described from a polyphasic approach. Agrociencia Uruguay 24, https://doi.org/10.31285/agro.24.52
Pérez, M. d. C. 2002. Fitoplancton del río Negro, Uruguay. Limnetica 21: 81-92.
Pérez, M. d. C., S. Bonilla & L. De Leôn, J. Šmarda & J. Komárek. 1999a. A bloom of Nodularia baltica-spumigena group (Cyanobacteria) in a shallow coastal lagoon of South America. Algological Studies/Archiv für Hydrobiologie, Supplement 93: 91-101. https://doi.org/10.1127/algol_stud/93/1999/91
Pérez, M. d. C., S. Bonilla & G. Martínez. 1999b. Phytoplankton community of a polymictic reservoir, La Plata River basin, Uruguay. Revista Brasileira de Biologia 59: 535-541. https://doi.org/10.1590/s0034-71081999000400002
Pérez, M. d. C. & A. Carillo. 2005. Picocyanobacteria distribution in the Ebro estuary (Spain). Acta Botanica Croatica 64: 237-246.
Pérez, M. d. C., A. Comas & N. I. Maidana. 2010. Estudio taxonómico del fitoplancton del tramo inferior del río Júcar con especial énfasis en las algas verdes cocales (Valencia-España). Algas 44: 13-19.
Pérez, M. d. C., N. I. Maidana & A. Comas. 2009. Phytoplankton composition of the Ebro River estuary, Spain. Acta Botanica Croatica 68: 11-27.
Pérez, M. d. C. & C. Odebrecht. 2005. The phytoplankton structure of Merin Lagoon: a Subtropical World Biosphere Reserve System (Brasil–Uruguay). Acta Botanica Croatica 64: 247–261.
Piccini, C., L. Aubriot, A. Fabre, V. Amaral, M. González-Piana, A. Giani, C. C. Figueredo, L. Vidal, C. Kruk & S. Bonilla. 2011. Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae 10: 644-653. https://doi.org/10.1016/j.hal.2011.04.016
Porzekanski, B., J. C. Blanco & E. P. Moure. 1965. Algas agarígenas y agar del Uruguay. Anales de la Facultad de Medicina 50: 157-168.
Rigamonti, N., L. Aubriot, F. Martigani, S. Bonilla & C. Piccini. 2019. Effect of nutrient availability on cylindrospermopsin gene expression and toxin production in Cylindrospermopsis raciborskii. Aquatic Microbial Ecology 82: 105-110. https://doi.org/10.3354/ame01877
Roldán, G. 2020. Historical review of limnology in Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 44: 303-328. https://doi.org/10.18257/raccefyn.1056.
Santibañez, I. 1939. Contribución al conocimiento de las diatomeas uruguayas. Revista Sudamericana de Botánica 6: 6-9.
Segura, A. M., C. Kruk, D. Calliari & H. Fort. 2013. Use of a morphology-based functional approach to model phytoplankton community succession in a shallow subtropical lake. Freshwater Biology 58: 504-512. https://doi.org/10.1111/j.1365-2427.2012.02867.x
Sophia, M. D. G. & M. d. C. Pérez. 2010. Planktic Desmids from Merin Lagoon, a biosphere world reserve. Iheringia - Série. Botanica 65: 183-199.
Steinitz-Kannan, M., C. López, D. Jacobsen & M. de L. Guerra. 2020. History of limnology in Ecuador: a foundation for a growing field in the country. Hydrobiologia 847: 4191-4206. https://doi.org/10.1007/s10750-020-04291-1
Tornés, E., M. C. Pérez, C. Durán & S. Sabater. 2014. Reservoirs override seasonal variability of phytoplankton communities in a regulated Mediterranean river. Science of the Total Environment 475: 225–233. https://doi.org/10.1016/j.scitotenv.2013.04.086
Tundisi, G. & T. Matsumura-Tundisi. 2012. Limnology, CRC Press, Leiden.
Vélez-Rubio, G. M., L. González-Etchebehere, F. Scarabino, R. Trinchin, G. Manta, M. Laporta, M. Zabaleta, V. Vidal, A. de Leon-Mackey, & C. Kruk. 2021. Macroalgae morpho-functional groups in Southern marine ecosystems: rocky intertidal in the Southwestern Atlantic (33°35° S). Marine Biology 168: 153. https://doi.org/10.1007/s00227-021-03960-6
Vico, P., L. Aubriot, F. Martigani, N. Rigamonti, S. Bonilla & C. Piccini. 2016. Influence of nitrogen availability on the expression of genes involved in the biosynthesis of saxitoxin and analogs in Cylindrospermopsis raciborskii. Harmful Algae 56: 37-43. https://doi.org/10.1016/j.hal.2016.04.008
Vico, P., S. Bonilla, B. Cremella, L. Aubriot, A. Iriarte & C. Piccini. 2020. Biogeography of the cyanobacterium Raphidiopsis (Cylindrospermopsis) raciborskii: Integrating genomics, phylogenetic and toxicity data. Molecular Phylogenetics and Evolution 148. https://doi.org/ 10.1016/j.ympev.2020.106824
Sometido: 15 de diciembre de 2022.
Aceptado: 16 de diciembre de 2022.