Water quality assessment in the coastal zone of Campeche, southeastern Gulf of Mexico
Evaluación de la calidad del agua en la zona costera de Campeche, sureste del Golfo de México
Carlos Antonio Poot-Delgado1, Jaime Rendón-von Osten2, Yuri B. Okolodkov3* & Maurilio Lara-Flores2
1 Tecnológico Nacional de México / Instituto Tecnológico Superior de Champotón, Carretera Champotón-Isla Aguada Km 2, El Arenal, CP 24400 Champotón, Campeche, México
2 Instituto de Ecología, Pesquerías y Oceanografía del Golfo de México, Universidad Autónoma de Campeche, AP 520, CP 24030 Campeche, Campeche, México
3 Instituto de Ciencias Marinas y Pesquerías, Universidad Veracruzana, Mar Mediterráneo 314, Fracc. Costa Verde, CP 94294 Boca del Río, Veracruz, México
*Corresponding author: yuriokolodkov@yahoo.com
Poot-Delgado, C.A., Rendón-von Osten, J., Okolodkov, Y.B. & Lara-Flores, M. 2021. Water quality assessment in the coastal zone of Campeche, southeastern Gulf of Mexico. Cymbella 7 (3): 79-99. https://cymbella.mx.
ABSTRACT
The purpose of this study was to assess the water quality at several sites with anthropogenic impact in the southeastern Gulf of Mexico. Eight sites at 3 coastal localities with different kinds of anthropogenic activity were monitored in September and November 2016. Water temperature, salinity, pH, dissolved oxygen, inorganic nutrients, heavy metals in sediments, fecal coliforms and phytoplankton abundance were determined. The pH values and the dissolved oxygen suggested a predominance of photosynthetic activity. Elevated nutrient contents were associated with sites of rainwater discharge with anthropogenic activities, as well as with biogeochemical processes. Inorganic nutrients, especially ammonium, nitrites and nitrates, heavy metals, and phytoplankton abundance showed influences of anthropogenic factors and can be considered indicators of water quality, although fecal coliforms (up to 900 Most Probable Number 100 mL-1) were not a good indicator of fecal contamination. The Nanoflagellates were the most abundant, followed by diatoms. The non-toxic diatom Cylindrotheca closterium, known to be a bloom-forming species, and the harmful cyanobacterium Trichodesmium sp. showed proliferations on the order of 105 cells L-1. These phytoplankton responses are indicative of the eutrophication due to fluctuations in environmental conditions because of the intensity and type of human activities that take place in the study area.
Keywords: eutrophication, Gulf of Mexico, heavy metals, phytoplankton, water qualityRESUMEN
El propósito de este estudio fue evaluar la calidad del agua en varios sitios con impacto antropogénico en el sureste del Golfo de México. En septiembre y noviembre de 2016 se monitorearon 8 sitios en 3 localidades costeras con diferentes tipos de actividad antropogénica. Se obtuvieron los datos de temperatura del agua, salinidad, pH, oxígeno disuelto, nutrientes inorgánicos, metales pesados en sedimentos. También se determinaron coliformes fecales y abundancia de fitoplancton. Los valores de pH y el oxígeno disuelto sugirieron un predominio de la actividad fotosintética. Los contenidos elevados de nutrientes se asociaron con sitios de descarga de agua de lluvia con actividades antropogénicas, así como con procesos biogeoquímicos. Los nutrientes inorgánicos, especialmente amonio, nitritos y nitratos, metales pesados y abundancia de fitoplancton mostraron influencias de factores antropogénicos y pueden ser considerados indicadores de la calidad del agua, aunque los coliformes fecales (hasta 900 Número Más Probable 100 mL-1) no fueron un buen indicador de contaminación fecal. Los nanoflagelados fueron los más abundantes, seguido de las diatomeas. La diatomea no tóxica Cylindrotheca closterium, una especie formadora de florecimientos y la cianobacteria nociva Trichodesmium sp. mostraron proliferaciones del orden de 105 células L-1. Estas respuestas del fitoplancton son indicativas de la eutrofización debido a las fluctuaciones en las condiciones ambientales propiciadas por la intensidad y tipo de actividades humanas que tienen lugar en el área de estudio.
Palabras clave: calidad del agua, eutrofización, fitoplancton, Golfo de México, metales pesados.INTRODUCTION
In Mexico, in general, water quality criteria for the protection of aquatic life in estuarine and coastal environments have ignored the benefits of the coastal ecosystem health indicators used in other regions. The information published in Mexican regulations is very limited, and the standards are focused on the beaches. Since 2003, in Mexico, through the Integral Program of Clean Beaches, the monitoring of seawater quality has been carried out following the criteria described by the World Health Organization (WHO) for recreational sites within 17 coastal states of the country. Given the technical and economic difficulties in determining all the parameters related to the deterioration of water quality, the fecal enterococcal content was used as an indicator of the degree of contamination of seawater and the health risks resulting from its recreational use (SEMARNAT 2014).
For many decades, fecal coliform counts have been performed in Mexican coastal waters; thus, the value of the historical data is unquestionable. Fecal coliforms (FC) are widely recognized as bacteriological indicators because their presence in aquatic systems is evidence of fecal contamination. Under current Mexican regulations, Enterococcus spp. are an indicator for establishing the microbiological quality of a beach for recreational use (SE 2016). However, the use of a single parameter can lead to erroneous results when characterizing a water body by only considering the potential danger of pathogens (Escobedo-Urías et al. 1999); therefore, a complex of variables is usually used.
Phytoplankton can be useful for the trophic classification of the aquatic environment as they reflect environmental conditions, respond quickly to changes that may occur through natural processes or human activities and modify the structure of their communities (De la Lanza-Espino & Gómez-Rojas 2005; Poot-Delgado et al. 2022). The dominant species, their relationships with environmental changes and their biomass increase can be used as indicators of the varying characteristics of coastal water quality as well as to predict the possible changes that could occur in the upper levels of trophic food webs (De la Lanza-Espino et al. 2000).
In recent years, many environmental agencies around the world have used methods for assessing water quality based on biological communities (Ospina-Álvarez & Peña 2004). The regulatory framework of the European Directive that incorporates phytoplankton into the definition and classification of the ecological status of aquatic bodies, using metrics such as biomass (chlorophyll-a), changes in the community (species composition and abundance) and increased frequency and intensity of blooms is an example (Revilla et al. 2009). The standard Mexican NMX-AA-120-SCFI-2006 (SE 2016) does not consider the study of microalgal communities as one of the methodological lines to be followed for the characterization of different types of lakes, lagoons, reservoirs or coastal water bodies to obtain metrics for evaluation of their ecological state.
Sediments are also valuable indicators for monitoring pollutants in aquatic environments. They act as traps for various types of toxic substances, such as traces of accumulated heavy metals from direct or indirect discharges through effluents, runoff and leachates originating from numerous urban, industrial and agricultural activities, as well as from atmospheric deposition (Bejarano-Ramírez et al. 2017). It should be noted that there is no official Mexican standard for the maximum permissible limits of heavy metals in coastal sediments.
Since each ecosystem is unique, it is necessary to use effective tools for diagnosis and to identify the key parameters that indicate the degree of human impact or ecological status (Crooks & Turner 1999). To do this, the components of the medium that can be used as indicators of ecological quality should be determined; these can be physicochemical (temperature, salinity, total suspended solids, dissolved oxygen (DO), nutrients), hydromorphological (tides, waves, depth, currents) or biological variables (phytoplankton, benthic flora and fauna) (Hermosilla-Gómez 2009).
For this purpose, partial information on the phytoplankton abundances and environmental variables (temperature, salinity, pH and DO) for September and November 2016 has been recovered from the data published in Poot-Delgado et al. (2022), where the authors concluded that the composition of the phytoplankton community, harmful and bloom-forming species may be subject to the increasing anthropogenic impact in the coastal zone of the State of Campeche. The purpose of the present study was to assess the water quality and to test the previous hypothesis with a greater number of variables (also considering inorganic nutrients, heavy metals in sediments and fecal coliforms).
MATERIAL AND METHODS
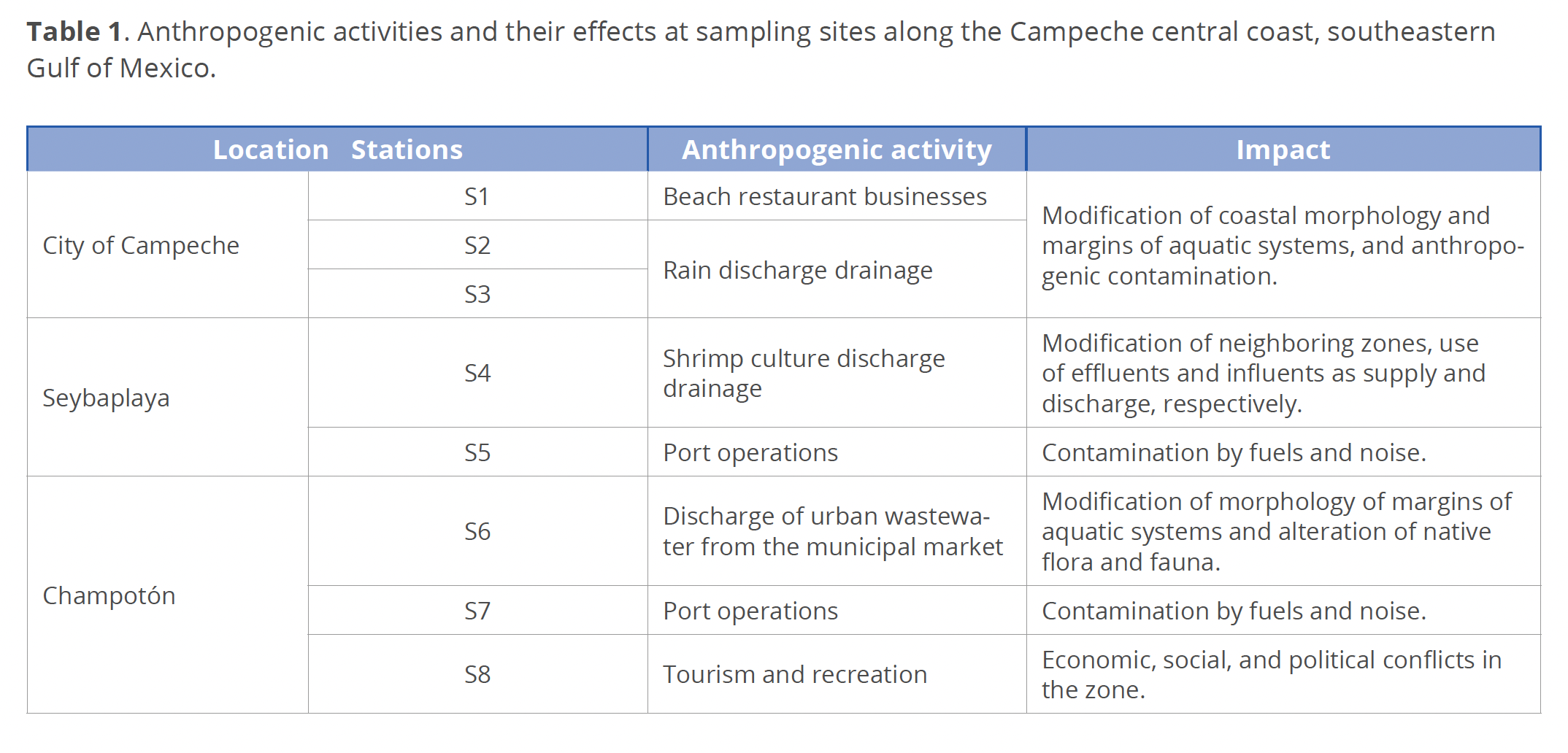
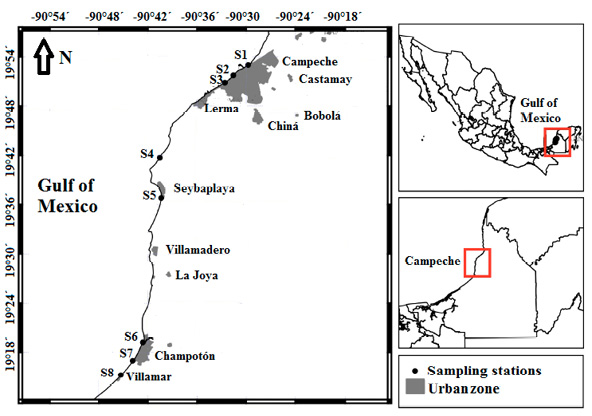
Field sampling. Sampling was carried out on September 10 and November 14, 2016, corresponding to the end of the rainy season (June to September) and the beginning of the windy season (October to January), respectively, at 3 coastal localities in the central zone of the State of Campeche (Table 1).
The average monthly rainfall in September was 202.8 mm; in November, it was 53.4 mm (CONAGUA 2016). In the study region the wind blows from the west almost throughout the year, causing a persistent westward circulation (Zavala-Hidalgo et al. 2003; Kurczyn et al. 2020). From September to June, atmospheric cold fronts considerably change the regional wind flow and interrupt upwelling processes from the northeast (Kurczyn et al. 2021). Sampling stations (st.) were chosen at the points of rainwater discharge or near the sites with anthropogenic activities (Table 1, Fig. 1). Site depths were approximately 1 m at all the stations.
Surface seawater samples were collected with 1-liter plastic bottles; an aliquot of 100 mL was used for cell counting of phytoplankton taxa. Samples were immediately fixed with an alkaline iodine solution and subsequently preserved by adding 37% neutralized formalin to a final concentration of 4% (Throndsen 1978). Additionally, horizontal tows were taken for 5 min. with a conical hand net of 20 μm mesh size. The collected material for the study of phytoplankton was placed in glass vials and fixed using the same procedure as for cell counts. In situ temperature (°C), salinity, pH, and DO were measured using a HANNA Multiparameter probe, model HI9828, with a sensor model HI769828 (Hanna Instruments Inc., Woonsocket, RI, USA) and a HACH Multiparameter probe, model HQ40d (Hach Company, Loveland, CO, USA).
For analysis of fecal coliforms (FC), water samples were collected in sterile Pyrex 250 mL flasks at each sampling site and were cold transported for later laboratory analysis.
To analyze the concentrations of the heavy metals aluminum (Al), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), manganese (Mn), lead (Pb), vanadium (V) and zinc (Zn), sediment samples were collected from the muddy bottom (to a depth of 15 cm) in nursery bags and preserved in ice at 4 °C for further analysis. For processing the sediment matrix, the samples were dried in an oven at 35 °C for 48 h.
Laboratory analyses. Orthophosphate, ammonium, nitrite, nitrate and silicate analyses were performed in the laboratory following standard chemical methods for marine environmental monitoring (UNEP 1991). Phytoplankton cell counting was performed according to Utermöhl (1958), using 10-ml sedimentation cylinders and a Zeigen inverted microscope equipped with phase contrast 10x/0.25 Ph1 ADL and LD 25x/0.30 Ph1 objectives. Nanoflagellates (<20 µm), due to their small size, were not identified to species or higher level, nor were autotrophic and heterotrophic nanoflagellates distinguished. Abundance values were expressed as cells L-1. Phytoplankton species were identified in fixed samples with a Carl Zeiss Axiostar Plus compound microscope (Oberkochen, Germany) equipped with 5x/0.10, 10x/0.25, 20x/0.40, 40x/0.65, and 100x/1.25 planachromatic objectives. Specialized literature was consulted to identify phytoplankton species: Schiller (1931), Cupp (1943), Osorio-Tafall (1942), Dodge (1982), Komárek & Anagnostidis (1986a, b), Fukuyo et al. (1990).
The determination of FC was in accordance with the standard NOM-112-SSA1-1994 (SSA 1994) that consists of a presumptive and confirmatory test; 0.1, 1 and 10 mL of each sample were inoculated in triplicate in 25 mL tubes with 10 mL of lactose broth with Durham tubes inverted inside and were incubated at 36 °C for 24 h. From the tubes that were positive for gas production, aliquots were taken and inoculated into tubes with 2% brilliant green bile broth containing inverted Durham tubes and incubated at 36 °C for 24 h for quantifying FC. The microbial density is expressed as a most probable number (MPN) per 100 mL.
Metal contents in sediment were determined by differential pulse anodic stripping voltammetry (DPASV) following the procedure proposed by Praveen-Kumar et al. (2005): 0.5 g of sediment was transferred to a Teflon reaction vessel with 10 mL of distilled water and an acid mixture of 5 ml of 65% HNO3, 4 ml of HF at 38-40%, and 1 mL of 37% HCl. The digestion was carried out in a microwave digestion system MARS Xpress 5 (CEM Corporation) (USEPA 1996). The digested sample was completed to a volume of 250 mL, and the pH was adjusted to 2.2 with 0.1 M NaOH. Voltammetric measurements of heavy metals were performed with a 797 VA Computrace (Metrohm AG) electrochemical system and computer-controlled by VA Computrace Metrodata software (Metrohm AG). The techniques for determination of the heavy metals were performed according to the USEPA (1996). Validation and quality control according to Metrohm standard application procedures were used in each metal determination. Similarly, the calibration curves of each of the metals analyzed were performed with certified standards.
Statistical analyses. To observe changes in physicochemical variables, the normality of the recorded data was assessed with the Kolmogorov-Smirnov test and homoscedasticity with the Bartlett’s test (Garson 2012). The hypotheses of differences between seasons were tested by analysis of variance (ANOVA), and Tukey TSD (truly significant difference) was applied with a significance level of 0.05 (Daniel 1993). Calculation routine was performed with Statgraphics Centurion XV program (version 18.2.06). SigmaPlot (version 10.0) was used to obtain graphics of phytoplankton and physicochemical variables (Statgraphics Technologies, Inc., The Plains, VA, USA).
The effect of physicochemical variables on FC and abundances of a major group of phytoplankton was tested using canonical correspondence analysis (CCA). It was performed using a matrix of environmental factors and abundances of a major group of phytoplankton (Ter Braak 1986). Data were transformed to Log 10 (data + 1) before the analysis because (1) data did not follow a normal distribution, and (2) a significant difference in magnitude between the values of biological and physicochemical data occurred. The significance of the axes of the CCA was tested using a Monte Carlo analysis with 199 permutations (Ter Braak 1986). Calculation routine was performed using the CANOCO program (version 201.4.56).
RESULTS
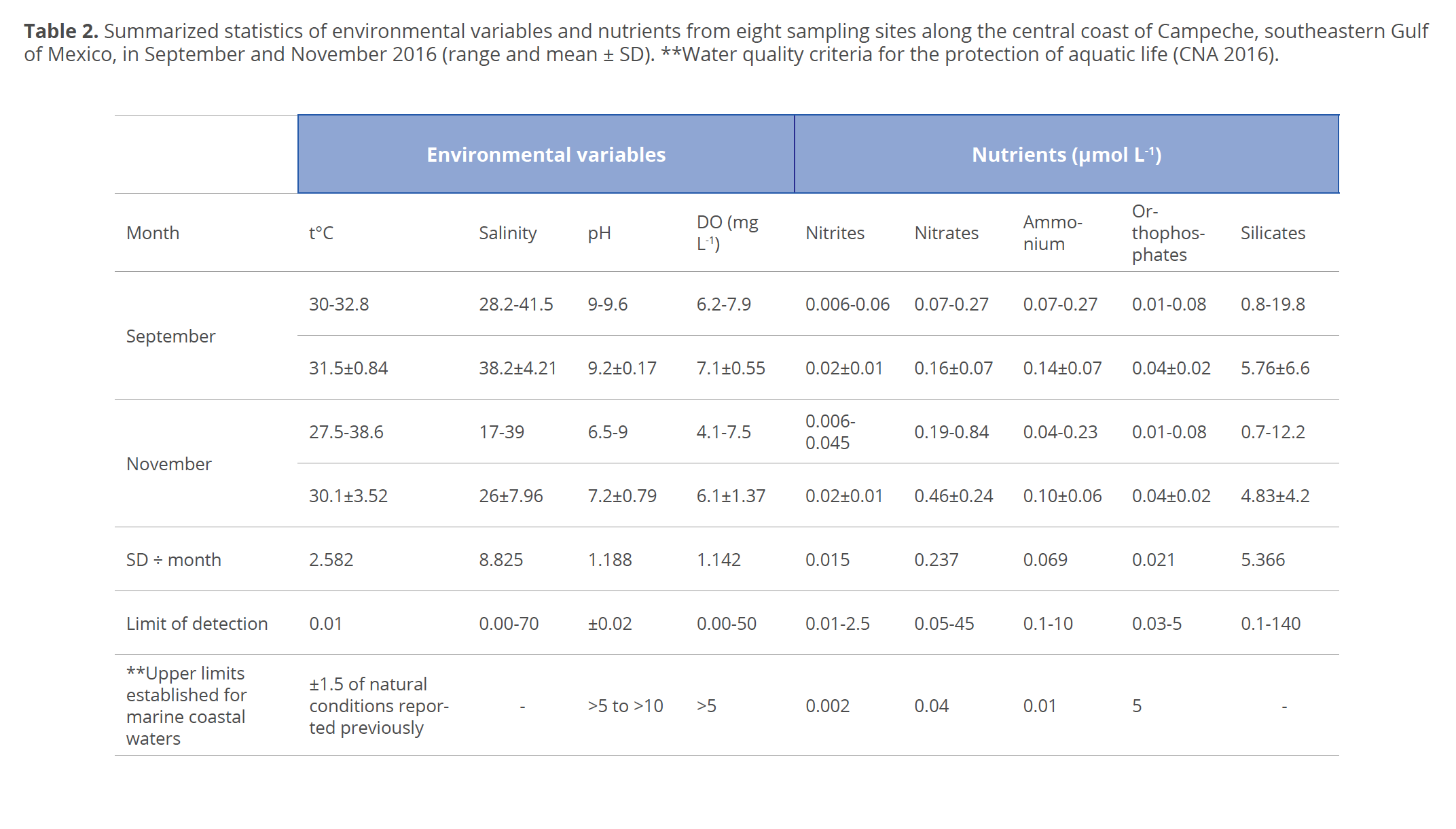
The results obtained from both physicochemical and bacteriological characteristics were compared with the water quality criteria of the maximum permissible limits established for estuaries and coastal marine waters in monthly averages under Mexican laws (SEMARNAT 1996; CNA 2016). The variability of physicochemical characteristics is shown in Figures 2 and 3 and in Table 2.
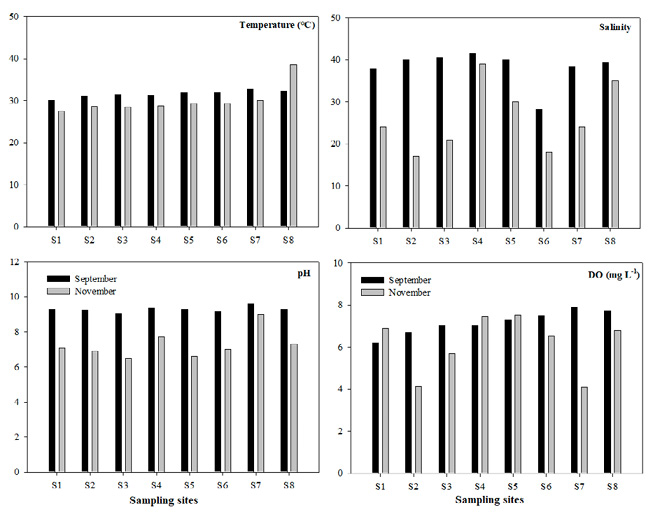
Temperature. The eight stations sampled in September showed an average of 31.5 °C, a minimum of 30 °C at st. 1 and a maximum of 32.8 °C at st. 7. In November, the eight stations showed an average of 30.1 °C with a variability range of ±3.52 °C, a maximum of 38.6 °C at st. 8 and a minimum of 27.5 °C at st. 1. However, there were no significant differences between months (F=0.001; p>0.05).
Salinity. The salinity in September showed an average of 38.25 with a variability range of ±0.21, a minimum of 28.2 at st. 6 and a maximum of 41.5 at st. 4. In November the eight stations showed an average of 26 with a variability range of ±7.96, a minimum of 17 at st. 2 and a maximum of 39 at st. 4 (Fig. 2). There were no significant differences between months (F=0.11; p>0.05).
pH. The pH values at the eight stations in September showed an average of 9.2 and a standard deviation of 0.17, with a variability range of ±0.5. In November, the pH values fluctuated ±2.5 with an average of 7.2 and a standard deviation (SD) of 0.79, with a maximum of 9.0 at st. 7 and a minimum of 6.5 at st. 3 (Fig. 2). Significant differences occurred between months (F=0.0005; p<0.05).
Dissolved oxygen. The values of DO at the eight stations in September showed an average of 7.1 mg L-1 with a variability range of ±0.55. In November, the minimum value was 4.1 mg L-1 at st. 7, the maximum value was 7.5 mg L-1at st. 5 and the average value was 6.1 mg L-1 with a variability range of ±1.37 (Fig. 2). DO showed no significant differences between months (F=0.02; p>0.05).
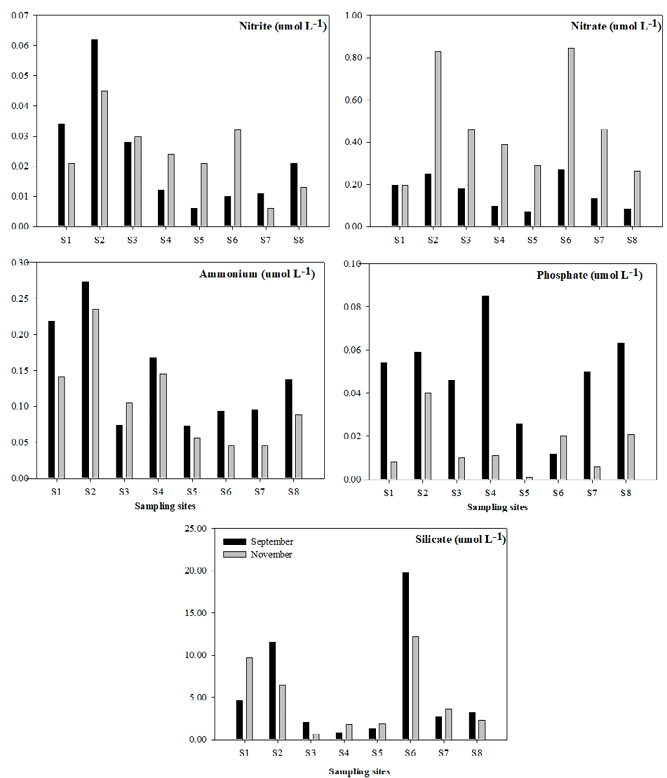
Nitrogen-containing nutrients. In September, at all stations nitrites were above the maximum limit established for marine waters (Table 2). As in November, the maximum value of 0.045 μmol L-1 was determined at st. 2 (Fig. 3). There were no significant differences observed between months (F=0.27; p>0.05). In September and November, nitrates were above the maximum limit established for marine waters. There were significant differences between months (F=0.006; p<0.05). In September, at st. 1, 2, 4 and 8 ammonium was above the maximum limit established for marine waters. In November, only st. 1, 2, 3 and 4 showed values above the maximum limits established for marine waters. Significant differences occurred between months (F=0.074; p<0.05).
Orthophosphates. In both September and November, orthophosphates were well below the maximum limit established for marine waters (Table 2). No significant differences were observed between months (F=1; p>0.05).
Silicates. In September, silicates did not differ significantly between months (F=0.25; p>0.05). It should be noted that silicates are not included in the current Mexican regulations (Table 2).
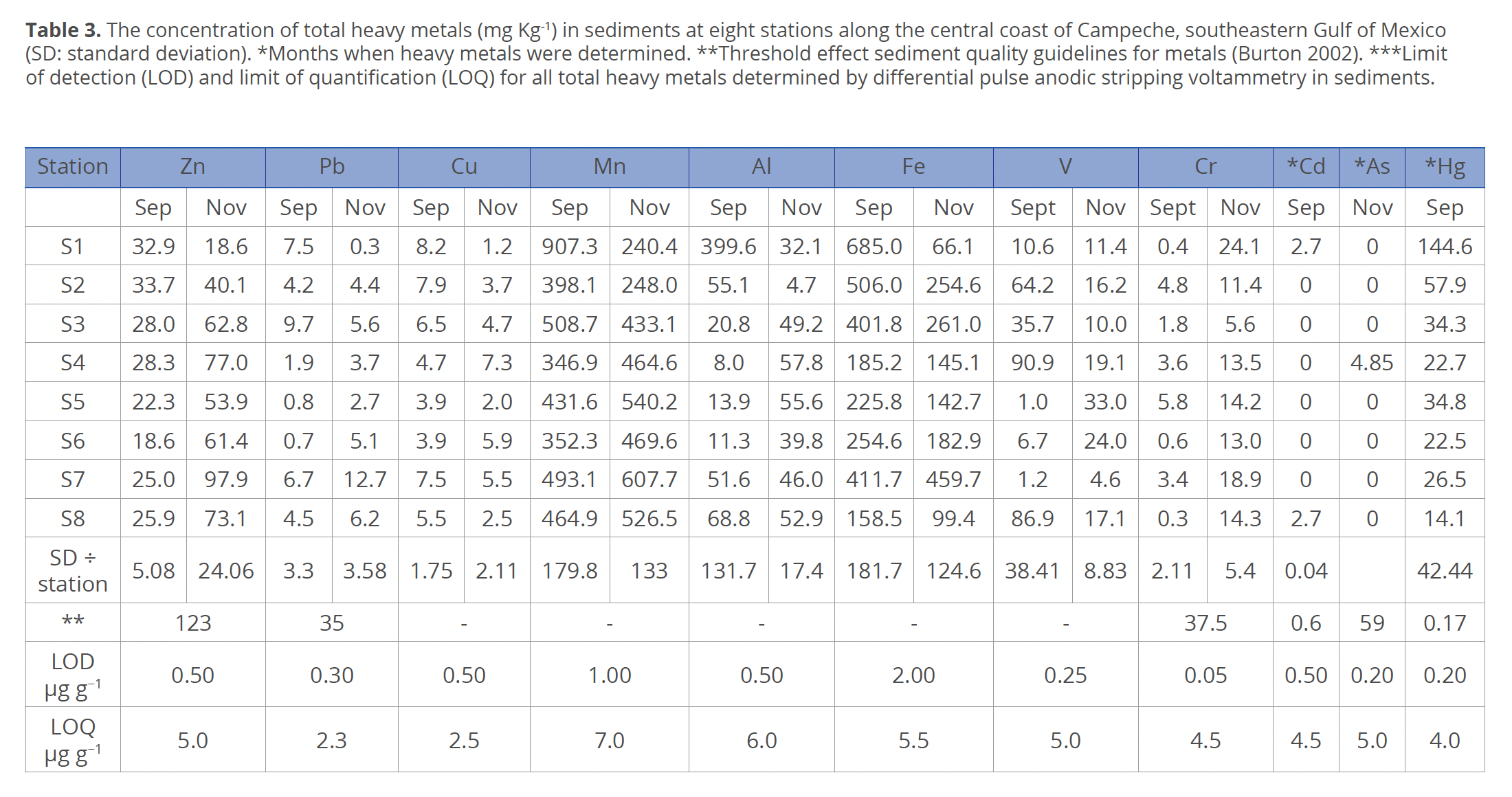
Total heavy metals. The total concentration of total heavy metals (Table 3) varied widely between months. Only Zn showed significant differences between months (F=0.044; p<0.05). Cu, Fe and Hg showed the highest values in September, and Zn, Pb, Mn and Cr in November. Al and V showed maximum and minimum values in September. Mn was the highest in September at st. 1. Cd was present in September at st. 1 and 8, and As was present at st. 4 in November.
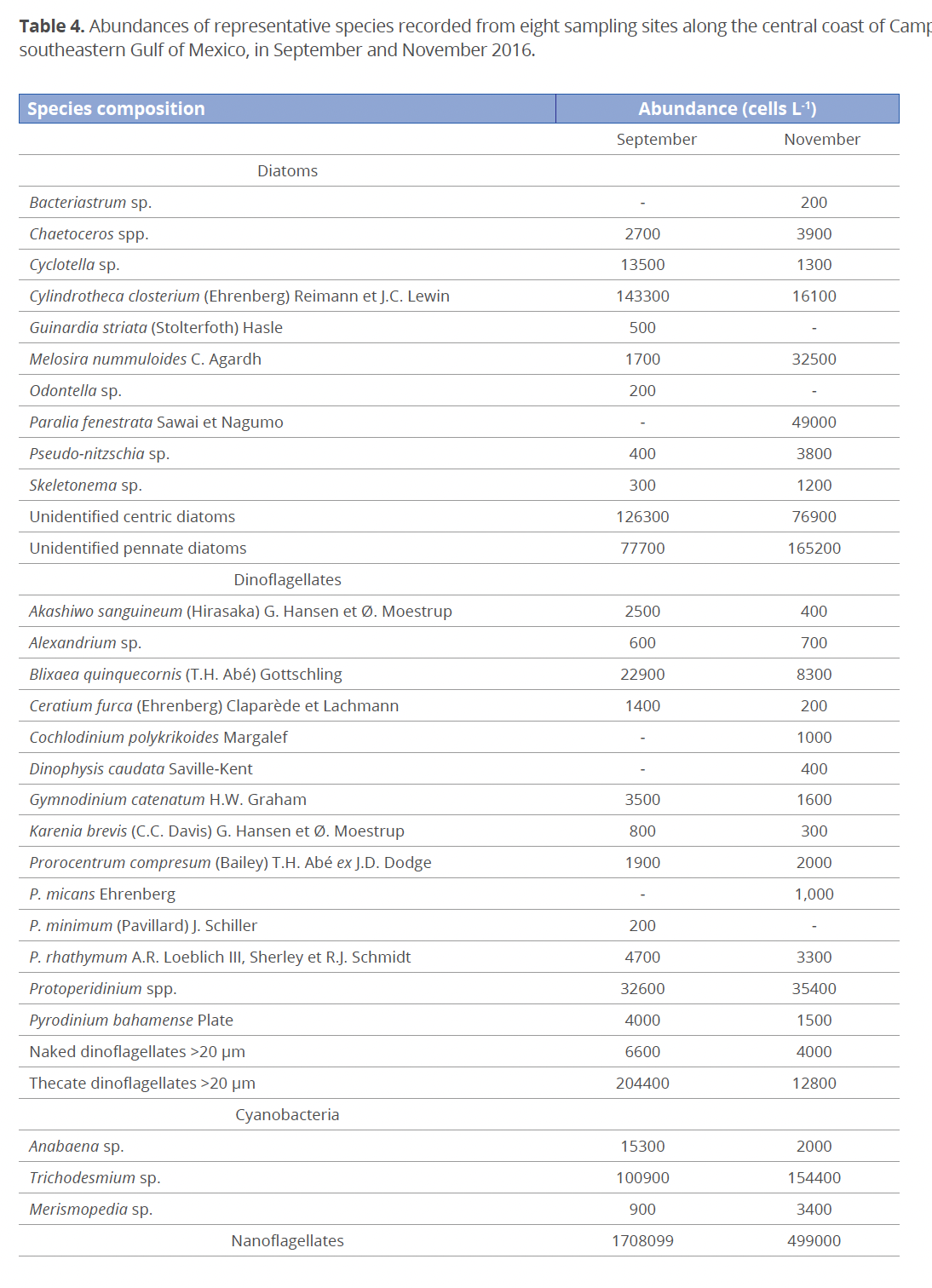
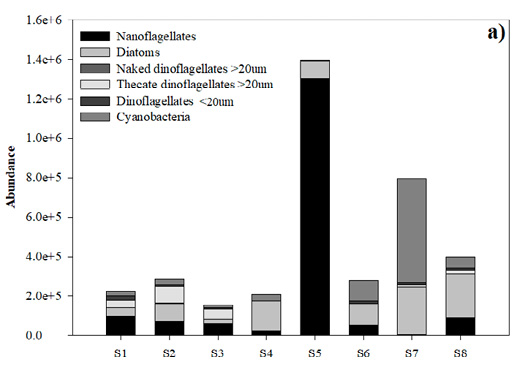
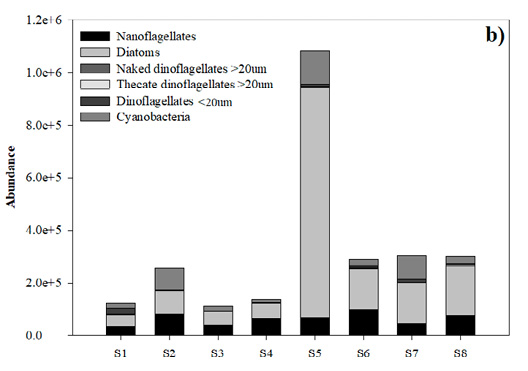
Phytoplankton in general. In total, 43 microalgal species were identified, of which 18 (41%) were diatoms, 17 (39%) dinoflagellates, 4 (10%) cyanobacteria, 3 (7%) nanoflagellates and 1 (3%) silicoflagellate (Table 4). The average abundance of marine phytoplankton during the study period was 4.8×105 cells L-1, ranging from 1.1×105 to 1.5×106 cells L-1 (Fig. 4a, b). In September, the minimum value of 6.0×103 cells L-1 and the maximum value of 1.3×106 cells L-1 between stations (with a mean of 1.53×105 cells L-1 and a SD of 4.8×105 cells L-1) were recorded; unidentified nanoflagellates were the most abundant at st. 5. At all the other stations dinoflagellates were the most abundant, followed by diatoms. Cyanobacteria showed their maximum abundance at st. 7 (Fig. 4a). In November, a minimum value of 1.1×105 cells L-1 and a maximum value of 1.2×106 cells L-1 (with an average of 3.7×105 cells L-1 and a SD of 3.5×105 cells L-1) were observed; diatoms were the dominant taxonomic group (Fig. 4b). In September and November the minimum total phytoplankton abundance occurred at st. 3 and the maximum at st. 5. There were no significant differences between months (F=0.42; p>0.05).
Nanoflagellates. In September, the highest nanoflagellate abundance was observed at st. 5 (1.31×106 cells L-1) and the minimum value at st. 7 (6.0×103 cells L-1), with a mean of 2.1×105 cells L-1 and a SD of 4.4×105 cells L-1 (Fig. 4a). In November, the total abundance, in general, was lower; it decreased markedly to 3.3×104 cells L-1 at st. 1, and a maximum value of 9.7×104 cells L-1 occurred at st. 6, with a mean of 6.2×104 cells L-1 and a SD of 2.2×104 cells L-1 (Fig. 4b). There were no significant differences between months (F=3.85; p>0.05).
Diatoms. In September, the average diatom abundance was 1.1×105 cells L-1 with a SD of 7.8×104 cells L-1. The minimum value (1.8×104 cells L-1) was recorded at st. 3 and the maximum (2.3×105 cells L-1) at st. 7 (Fig. 4a). In November, the maximum value (8.7×105 cells L-1) was recorded at st. 5 and the minimum value (4.5×104 cells L-1) at st. 1, with a mean of 2×105 cells L-1 and a SD of 2.7×105 cells L-1 (Fig. 4b). However, no significant differences were observed between months (F=0.003; p>0.05). Cylindrotheca closterium was the most abundant diatom with a maximum value of 1.4×105 cells L-1. Its maximum abundance was observed in September at st. 3.
Naked dinoflagellates >20 µm. In September, the average naked dinoflagellate >20 µm abundance was 950 cells L-1 with a SD of 1.2×103 cells L-1. A minimum value (400 cells L-1) was recorded at st. 6 and a maximum (3.0×103 cells L-1) at st. 2 (Fig. 4a). In November, the maximum value (1.3×103 cells L-1) was recorded at st. 8 and a minimum value (400 cells L-1) at st. 7, with a mean of 475 cells L-1 and a SD of 582 cells L-1 (Fig. 4b). However, no significant differences were observed between months (F=1.01; p>0.05).
Thecate dinoflagellates >20 µm. In September, the highest thecate dinoflagellate >20 µm abundance was observed at st. 2 (8.1×105 cells L-1) and the minimum at st. 6 (500 cells L-1), with a mean of 2.5×104 cells L-1 and a SD of 2.9×104 cells L-1 (Fig. 4a). In November, the total abundance, in general, was lower; it decreased markedly to 100 cells L-1 at st. 7, and a maximum value of 5×103 cells L-1 occurred at st. 8, with a mean of 1.5×103 cells L-1 and a SD of 1.7×103 cells L-1 (Fig. 4b). Significant differences were observed between months (F=5.29; p<0.05).
Dinoflagellates <20 µm. In September, the minimum value (1.3×103 cells L-1) was recorded at st. 4 and the maximum value (2.1×104 cells L-1) at st. 1, with a mean of 9.3×103 cells L-1 and a SD of 5.9×103 cells L-1 (Fig. 4a). In November, the abundances were lower. The minimum value (1.0×103 cells L-1) was recorded at st. 2 and the maximum value (2.1×104 cells L-1) at st. 1, with a mean of 7.0×103 cells L-1 and a SD of 7.2×103 cells L-1 (Fig. 4b). No significant differences were observed between months (F=0.52; p>0.05). The non-toxic benthic-planktonic brackish-marine thecate dinoflagellate Blixaea quinquecornis (T.H. Abé) Gottschling (= Peridinium quadridentatum T.H. Abé, Peridinium quadridentatum (F. Stein) G. Hansen) was found in September with a maximum abundance of 6.5×103 cells L-1 at st. 3.
Cyanobacteria. Cyanobacteria ranged from none observed in September at st. 5 to a maximum value of 5.2×105 cells L-1 at st. 7, with a mean of 9.8×104 cells L-1 and a SD of 1.7×105 cells L-1 (Fig. 4a). In November, the minimum value (1.1×104 cells L-1) occurred at st. 4 and the maximum value (1.7×105 cells L-1) at st. 5, with a mean of 5.0×104 cells L-1 and a SD of 4.2×104 cells L-1 (Fig. 4b). There were no significant differences between months (F=0.001; p>0.05). The genus Trichodesmium Ehrenb. ex Gomont was present in both months and at all stations with values on the order of 105 cells L-1.
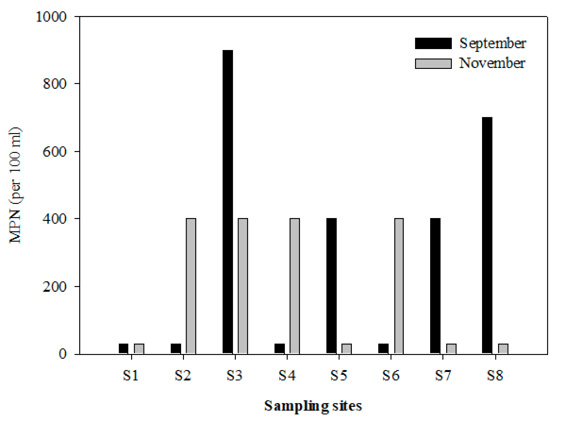
Fecal coliform bacteria. The highest concentrations of FC in September (>200 MPN per 100 mL) were observed at st. 5, 7 and 8, exceeding 900±344 MPN/100 mL at st. 3 (Fig. 5). This value exceeds that allowed for water quality according to the aquatic life protection ecological criteria (CNA 2016). Significant differences were observed between stations (p<0.05). In November, low levels of FC were found (Fig. 5) except at st. 2, 4, and 6 where values of >200 MPN 100 mL-1 were recorded. Significant differences were observed between stations (p<0.05). In both months, st. 1 showed a minimum value of <30 MPN 100 mL-1. However, no significant differences were observed between months (F=3.03; p>0.05).
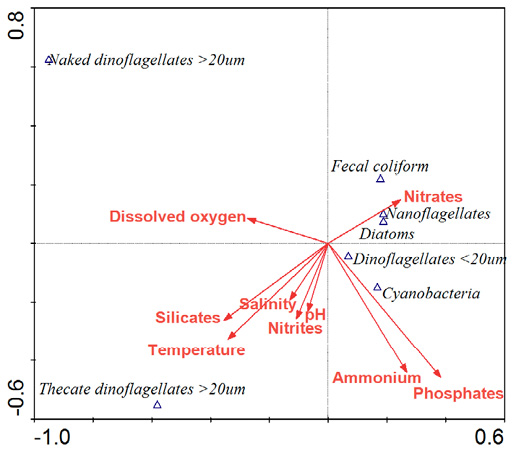
Relationships between bacteria, phytoplankton, and physicochemical characteristics. The response of the major phytoplankton groups to physicochemical variables is primarily explained by the first two axes (axis 1: 57.5%, axis 2: 34%, total 91.5%). The correlation between FC, major phytoplankton groups and physicochemical variables was high (r≈0.8), indicating a significant correlation between FC and phytoplankton species composition used in the analysis. All canonical axes were not statistically significant (p>0.05, Monte Carlo). However, the CCA graphs showed that the first axis (positive values) separates the samples of FC, nanoflagellates, diatoms, dinoflagellates <20 µm and cyanobacteria with high values of nitrites, ammonium, and orthophosphates from the samples with naked and thecates dinoflagellates >20 µm. Regarding the second axis FC, nanoflagellates and diatoms were abundant in positive coordinates when the nitrate concentration was higher. Cyanobacteria were abundant in negative coordinates, coinciding with the samples with high values of nitrites, ammonium, orthophosphates, and other physicochemical variables. However, the naked and thecated dinoflagellates >20 µm did not show an affinity for the measured physicochemical parameters (Fig. 6).
DISCUSSION
Physicochemical variables. The temperature and salinity at the sampled sites were shown to be favorable with respect to the presence and abundance of the major taxonomic phytoplankton groups. This is supported by the CCA analysis that showed a high correlation (r≈0.8). The ranges of the measured parameters varied depending on the climatic season caused by local hydrography and the shallowness of the study area (Poot-Delgado et al. 2014, 2022; Kurczyn et al. 2021). The pH values and oxygen saturation suggest high activity of the primary producers in the water column, leading to changes in water quality (especially in pH and DO) that could be related to the phytoplankton proliferations that occur in the area (Gómez-Figueroa 2020; Poot-Delgado et al. 2022).
Nitrogen compounds (nitrites, nitrates, and ammonium) were above the maximum limit established for marine waters, coinciding with those reported by Alpuche-Gual (2014), Varona-Cordero et al. (2014) and Gómez-Figueroa (2020). This is due to the effect of water discharges in the rainy season and to a lesser extent to the effect of sediment resuspension in the windy season (Monreal-Gómez et al. 1992, 2004; Varona-Cordero et al. 2014). The foregoing may suggest problems due to nutrient enrichment in the coastal waters of the Campeche central zone. For example, the presence of urban wastewater generates lower levels of DO due to bacterial degradation of organic matter, creating high negative redox potential values resulting in low levels of nitrites and high ammonium values (Masters & Ela 2008).
Silicates are not regulated within the water quality criteria for the protection of aquatic life; however, their values reported in this study are similar to those by Gómez-Figueroa (2020) who found the lowest concentrations at coastal sites compared to freshwater ones, which may be due to the abundance of silicates in the Earth’s crust that come from the weathering and metamorphism of rocks (Cervantes-Sandoval et al. 2014).
The orthophosphate values well below the maximum limit established for marine waters, contrary to those reported by Alpuche-Gual (2014) and Gómez-Figueroa (2020), may be due to the differences in the systems of phosphate acquisition and storage capacities between phyto- and bacterioplankton, which affect their response to phosphorus increase (Talarmin et al. 2015; Barcelos e Ramos et al. 2017).
Phytoplankton and physicochemical characteristics. A rather quick response of the phytoplankton community (in terms of the abundance and changes in the species composition) to eutrophication should be considered an ecological process, characterized by the complexity and peculiarities of each system (Barcelos e Ramos et al. 2017). In addition, each nutrient has a limiting potential that depends on many factors, such as the nutrient absorption rates in different major phytoplankton groups and interactions between the factors (Saito et al. 2008). Although this study represents a temporal section with its corresponding limitations (especially temporal), the results obtained can be considered as a photograph of the moment of the interaction of the abiotic and biotic variables of the water column to assess the water quality. The phytoplankton acts as a natural indicator of the effects of eutrophication, due to his rapid response to fluctuations of environmental conditions (Livingston 2001) because of the intensity and type of human activities (Kelly-Gerreyn et al. 2004; Álvarez-Góngora & Herrera-Silveira 2006).
In the study area, these conditions may contribute to changes in species composition and abundance of phytoplankton in general and nanoflagellates, diatoms, dinoflagellates <20 µm and cyanobacteria in particular. The latter two are likely to benefit from high temperatures (up to 30.1 °C) and the relatively low salinity (26) related to the continental runoff during the rainy season (Yáñez-Arancibia & Day 2005), which could be combined with the chemical compounds linked to urban residuals from adjacent settlements.
Phytoplankton cell size is important because smaller sized cells have an increased surface/volume ratio that provides a competitive advantage when nutrients are limiting (Chisholm 1992). Therefore, the nanophytoplankton and thecate dinoflagellate >20 µm abundance was high in September. No nitrogen limiting conditions can explain the predominance of small-sized species (cells >20 μm) whose growth would be supported by reduced forms of nitrogen such as ammonium. This is reinforced by the presence of the cyanobacterial genus Trichodesmium in both months throughout the study period, with abundances on the order of 105 cells L-1 due to the physiology of Trichodesmium spp., although they are non-heterocystous nitrogen-fixing species that contribute nitrogen in both oligotrophic (Holl et al. 2007) and eutrophic waters (White et al. 2007).
It is well accepted that human activities, especially nutrient pollution, have contributed to the global expansion of harmful algal blooms in terms of increasing abundance, frequency and geographic extent, and that new records of known species are being documented in new areas (Glibert & Burford 2017; Glibert 2020).
The benthic-planktonic pennate diatom Cylindrotheca closterium has been reported as a bloom-forming species in the southeastern Gulf of Mexico (Poot-Delgado 2016). In the coastal waters of northern Yucatan, C. closterium showed a high abundance throughout the year, so its seasonality remains unclear, although the highest abundances were found from mid-May to mid-October (Merino-Virgilio et al. 2019), coinciding with those observed during the study period.
In coastal brackish-water lagoons, the relative contribution of cyanobacteria to the total phytoplankton biomass can be used as an indicator of eutrophication (Poot-Delgado et al. 2018). It is known that an increase in temperature (>20 °C), favoring cyanobacteria blooms in marine ecosystems, is closely associated with eutrophication (Anderson et al. 2002). Trichodesmium spp. showed values on the order of 105 cells L-1 in both months, resulting in an alert for the occurrence of blooms of Trichodesmium in the coastal zone. Some marine Trichodesmium spp., such as T. thiebautii Gomont ex Gomont, can be toxic to smaller crustaceans, and Trichodesmium blooms may have indirect effects on molluscan and public health due to significant changes in water quality (Gárate-Lizárraga & Muciño-Márquez 2012). There is also evidence of a highly potent neurotoxic compound isolated from a mixed T. thiebautii – T. erythraeum bloom that occurred in the east Caribbean (for references, see Landsberg 2002). The blooms of Trichodesmium are followed by blooms of the toxic dinoflagellate Karenia brevis (C.C. Davis) G. Hansen & Ø. Moestrup; this was reported by Lenes et al. (2001) for the coasts of Florida. However, this species was absent at almost all the stations in Campeche, with very low abundances at some stations during the study period. Karenia spp. have been reported in variable abundances along the littoral of the Gulf of Mexico from Veracruz to Yucatán (Aké-Castillo 2011; Merino-Virgilio et al. 2013; Muciño-Álvarez et al. 2015; Poot-Delgado et al. 2018).
Finally, in trying to understand the life strategies of individual species, it must be recognized that phytoplankton populations in general are partly determined by the amplitude and frequency of fluctuations in the dynamics of their limiting resources. HAB constitute only part of the more difficult tasks of controlling and managing water quality in aquaculture, freshwater, brackish and marine environments (Poot-Delgado & Okolodkov 2016).
Fecal coliform bacteria. Marine pollution along the central coast of Campeche comes from discharges of wastewater from various anthropogenic activities (Table 1) aggravated by the absence or inefficiency of water treatment plants in the large cities of the region (Gracia et al. 2014), as was verified by the presence of FC found in concentrations above the current Mexican regulations for coastal waters and estuaries (240 MPN 100 mL-1 FC). However, the abundance of FC is mainly regulated by the synergistic effect among sunlight, temperature, salinity, and pH (Figueroa-Zavala 2007), which was not statistically observed in the present study (all the canonical axes were not statistically significant).
Surprisingly, at st. 3, an unexpected value of 900 MPN 100 mL-1 was found, well below values reported by Rendón-von Osten & Lara-Flores (2015) for the same study area (Campeche and Champotón; Fig. 1). Those authors reported values higher than 2400 MPN 100 mL-1 during the rainy season (May to September), which reflects the high population density adjacent to the discharge zone as well as the lack of treatment of the city’s waste (Rendón-von Osten & Lara-Flores 2015). Both recorded values coincide with the rainy season when they are closely linked to the rainwater discharges.
In this study, different anthropogenic activities (urban waste, port operations, tourism and recreation, beach restaurant businesses, rain discharge drainage and shrimp culture discharge drainage) affected the sampling stations, and the FC concentrations also varied. FC were not a good indicator of fecal contamination because they disappeared quickly. However, FC are known to be highly abundant in the coastal area, and their persistence indicates a constant effluent of sewage that contributes large amounts of organic matter to the system (Arcos-Pulido et al. 2005).
Total heavy metals. The analyzed total heavy metals were selected because they have been previously reported for the study area. Furthermore, most of them are toxic to aquatic organisms (Villanueva & Botello 1998; Botello et al. 2004). In aquatic environments heavy metals occur in higher concentrations in sediments, and their presence in the coastal zone is due to the anthropogenic activities and certain point sources (Vázquez-Gutiérrez et al. 1988). For most heavy metals, runoff water is the dominant source for the marine environment, except for some elements such as Hg and Pb, whose atmospheric input is important, particularly in the coastal zone (Páez-Osuna 2005). In the present study, metals considered to be of terrigenous origin were present in both September and November. This is supported by Carranza-Edwards et al. (2015), who found sediments of recent terrigenous origin on the beaches of the southern Gulf of Mexico, suggesting that there may be a greater influence of anthropogenic factors in recent sediments.
The same situation occurred in the sediments of the mangrove ecosystem influenced by Laguna de Términos, where the contaminating elements were mainly of anthropogenic origin (De la Cruz-Landero et al. 2013). Although in the present study the measurements of heavy metals were obtained only in sediments, it can be stated that their accumulated concentrations were very high, which likely affected the phytoplankton species by inhibiting their growth and reducing their chlorophyll concentration, photosynthesis and cell respiration. This type of response has been observed in trials with aqueous solutions of dissolved Cd, Cu, and Hg in monospecific cultures of the marine planktonic green microalga Tetraselmis chuii Butcher (Cordero et al. 2005). The total heavy metals are retained in sediments most of the time, and a detailed understanding of the direct relationship between sediment and surrounding water and bioavailability of heavy metals to phytoplankton is still lacking (Kottuparambil et al. 2019).
CONCLUSIONS
Nutrient enrichment, high abundance of FC (up to 900 MPN/100 mL at st. 3), high phytoplankton abundances (on the order of 106 L-1 cells), the presence of potentially harmful (including toxic) microalgal species, the presence of cyanobacteria (mainly Trichodesmium sp.) and temporal changes in the phytoplankton community were considered for evaluation of the water quality. The non-toxic benthic-planktonic brackish-marine pennate diatom Cylindrotheca closterium, previously reported as a bloom-forming species in the southern Gulf of Mexico, reached an abundance of 1.4×105 cells L-1 in September 2016. At the end of the rainy season (September), at st. 3 that was directly affected by rainwater discharge, the physicochemical conditions favored the proliferation (6.5x103 cells L-1) of the non-toxic dinoflagellate Blixaea quinquecornis, known to be associated with eutrophic environments. The determination of phytoplankton abundance and the presence of the bloom-forming species were the most useful tools for evaluating anthropogenic effects on the studied marine coastal ecosystem.
Some inorganic nutrients (especially ammonium, nitrites, and nitrates) showed an association with the rainwater discharge sites or with those near anthropogenic activities. At present, there is no official Mexican standard for the maximum permissible limits of heavy metals in coastal sediments.
Therefore, the choice of ecological indicators for water quality studies should be the result of careful consideration and an understanding of the natural mechanisms that cause stress in aquatic environments. In the case of eutrophication, nutrient enrichment must be considered to cause changes in biodiversity, taking as a response changes in the species composition of the phytoplankton community. However, due to the complexity and variability of phytoplankton, changes in the composition and structure of the phytoplankton community are not axiomatic. To understand their dynamics and to obtain knowledge of the factors underlying these changes, more detailed autecological and annual phytoplankton community studies are necessary. Coastal zones are subjected to wide ranges of physicochemical and biological variables. The present study may serve a baseline monitoring to distinguish between natural conditions and anthropogenically impacted environment in the near future.
ACKNOWLEDGMENTS
We thank Martin Memije-Canepa, María E. Ku-Euan, Favian García-Rodríguez, and Nallely G. Sánchez-Beberaje for laboratory analyses of water samples. We also thank Marcia M. Gowing from Seattle, WA, USA, who kindly improved the writing style and the three anonymous reviewers for their valuable critical comments. Mauricio González-Jauregui is thanked for technical assistance with the map. The financial support given to the project “Fitoplancton marino potencialmente nocivo de la costa central de Campeche, México” 022.16-P04 (2016-2017; project leader: CAPD) by COSDAC-SEMS-SEP (Coordinación Sectorial de Desarrollo Académico de la Subsecretaría de Educación Media Superior de la Secretaría de Educación Pública, México) is gratefully acknowledged.
REFERENCES
Aké-Castillo, J.A. 2011. Temporal dynamics of Trichodesmium erythraeum (Cyanophyta) in the National Park “Sistema Arrecifal Veracruzano” in the Gulf of Mexico. Journal of Environmental Biology 32: 395-399.
Alpuche-Gual, L. (Coord.) 2014. Clasificación de playas campechanas para su manejo integral y desarrollo sostenible. Universidad Autónoma de Campeche, Campeche.
Álvarez-Góngora, C.C. & J. Herrera-Silveira. 2006. Variations of phytoplankton community structure related to water quality trends in a tropical karstic coastal zone. Marine Pollution Bulletin 52: 48-60. https://doi.org/10.1016/j.marpolbul.2005.08.006
Anderson, D.M., P.M. Glibert & J.M. Burkholder. 2002. Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25:704-726. https://doi.org/10.1007/BF02804901
Arcos-Pulido, M.P., S.L. Ávila de Navia, S.M. Estupiñán-Torres & A.C. Gómez-Prieto. 2005. Indicadores microbiológicos de contaminación de las fuentes de agua. NOVA–Publicación Científica 3: 69-79. https://doi.org/10.22490/24629448.338
Barcelos e Ramos, J., K.G. Schulz, M. Voss, Á. Narciso, M.N. Müller, F.V. Reis, M. Cachão & EB Azevedo. 2017. Nutrient-specific responses of a phytoplankton community: a case study of the North Atlantic Gyre, Azores. Journal of Plankton Research 39: 744-761. https://doi.org/10.1093/plankt/fbx025
Bejarano-Ramírez I., J.M. Jurado, R. Muñiz-Valencia, A. Alcázar, S.G. Ceballos-Magaña, A. Olivos-Ortiz & O. Rangel. 2017. Comparative study of As, Cd, Cu, Cr, Mg, Mn, Ni, Pb and Zn concentrations between sediment and water from estuary and port. International Journal of Environmental Science and Technology 14: 1333-1342. https://doi.org/10.1007/s13762-016-1235-5
Botello, A.V., F.S. Villanueva & L. Rosales. 2004. Distribución y contaminación de metales en el Golfo de México. In: M. Caso, I. Pisanty & E. Ezcurra. Comps. Diagnóstico ambiental del Golfo de México. Secretaría de Medio Ambiente y Recursos Naturales, INE, Instituto de Ecología, Harte Research Institute for Gulf of Mexico Studies, México, pp. 583-710.
Burton, Jr.G. 2002. Sediment quality criteria in use around the world. Limnology 3: 65-76. https://doi.org/10.1007/s102010200008
Carranza-Edwards, A., A.Z. Márquez-García, C.I. Tapia-González, L.L. Rosales-Hoz & M.A. Alatorre-Mendieta. 2015. Cambios morfológicos y sedimentológicos en playas del sur del Golfo de México y del Caribe noroeste. Boletín de la Sociedad Geológica Mexicana 67: 21-43.
Cervantes-Sandoval, A.E., E. Constanzo-Casillas & J. Gómez-Márquez. 2014. Análisis de la calidad de aguas naturales y residuales con aplicación a la microescala. Universidad Nacional Autónoma de México, Ciudad de México. 204 pp.
Chisholm, S.W. 1992. Phytoplankton size. In: P.G. Falkowski & A.D. Woodhead. Eds. Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp. 213-237.
CNA (Comisión Nacional del Agua). 2016. Ley Federal de Derechos. Disposiciones Aplicables en Materia de Aguas Nacionales 2016. Secretaría de Medio Ambiente y Recursos Naturales, Ciudad de México. 173 pp.
CONAGUA (Comisión Nacional del Agua). 2016. Precipitación a nivel nacional y por entidad federativa 2016. http://smn.cna.gob.mx/tools/DATA/Climatolog%C3%ADa/Pron%C3%B3stico%20clim%C3%A1tico/Temperatura%20
y%20Lluvia/PREC/2016.pdf
Cordero, J., M. Guevara, E. Morales & C. Lodeiros. 2005. Efecto de metales pesados en el crecimiento de la microalga tropical Tetraselmis chuii (Prasinophyceae). Revista de Biología Tropical 53: 325-330. https://doi:10.15517/rbt.v53i3-4.14408
Crooks, S. & R.K. Turner. 1999. Integrated coastal management: Sustaining estuarine natural resources. Advances in Ecological Research 29: 241-289.
Cupp, E.E. 1943. Marine plankton diatoms of the west coast of North America. Bulletin of the Scripps Institute of Oceanography 5: 1-238. https://escholarship.org/uc/item/922945w8
Daniel, W.W. 1993. Bioestadística. Base para el análisis de las ciencias de la salud. Edit. Limusa México, Ciudad de México.
De la Cruz-Landero, N., Á. Alderete-Chávez & S. Laffón-Leal. 2013. Acumulación de metales pesados en sedimentos del ecosistema manglar en laguna de Términos, Campeche, México. Foresta Veracruzana 15: 25-30.
De la Lanza-Espino, G. & J.C. Gómez-Rojas. 2005. Calidad de agua e indicadores fitoplanctónicos en tres ambientes acuáticos costeros al noroeste del Golfo de México. In: A.V. Botello, J. Rendón-von Osten & G. Gold-Bouchot. Eds. Golfo de México. Contaminación e impacto ambiental: diagnóstico y tendencias. Tomo 1. 3a ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México. Instituto Nacional de Ecología, Campeche, México. 565-574 pp.
De la Lanza-Espino, G., S. Hernández-Pulido & J.L. Carbajal-Pérez. 2000. Organismos indicadores de la calidad del agua y de la contaminación (bioindicadores). Edit. Plaza y Valdés, México.
Dodge, J.D. 1982. Marine dinoflagellates of the British Isles. HMSO, London.
Escobedo-Urías, D., M.T. Hernández-Real, N. Herrera-Moreno, A. Ulloa-Pérez & A. Chiquete-Ozono. 1999. Calidad bacteriológica del Sistema lagunar de San Ignacio-Navachiste, Sinaloa. Ciencia y Mar 3: 17-27.
Figueroa-Zavala, B. 2007. Contaminación de origen fecal en el corredor costero Barra de Tonameca - bahía de Puerto Ángel - La Mina, Oaxaca, México. Ciencia y Mar 11: 15-28.
Fukuyo, Y., H. Takano, M. Chihara & K. Matsuoka. 1990. Red tide organisms in Japan. In: R. Uchida. Ed. An illustrated taxonomic guide. Tokyo, Japan, pp. 33-65.
Gárate-Lizárraga, I. & R.E. Muciño-Márquez. 2012. Blooms of Trichodesmium erythraeum and T. thiebautii (Cyanobacteria, Oscillatoriales) in the Bahía de La Paz, Gulf of California. CICIMAR Oceánides 27: 61-64. http://dx.doi.org/10.37543/oceanides.v27i1.110
Garson, D. 2012. Testing statistical assumptions. Statistical Associates Publishing, Asheboro.
Glibert, P.M. 2020. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 91:101583. https://doi.org/10.1016/j.hal.2019.03.001
Glibert, P.M. & M.A. Burford. 2017. Globally changing nutrient loads and harmful algal blooms: recent advances, new paradigms, and continuing challenges. Oceanography 30: 58-69. https://doi.org/10.5670/oceanog.2017.110
Gómez-Figueroa, J.A. 2020. Fitoplancton como bioindicador de la calidad de agua: evaluación de ecosistemas acuáticos del Estado de Campeche. Tesis de Maestría. Universidad Autónoma de Campeche, Campeche. 115 pp.
Gracia, A., G.F. Vázquez, G. Enciso-Sánchez & H.M. Alexander-Valdés. 2014. Composición y volumen de contaminantes de las descargas costeras al Golfo de México. In: A.V. Botello, J. Rendón-von Osten, J.A. Benítez, G. Gold-Bouchot. Eds. Golfo de México. Contaminación e impacto ambiental: diagnóstico y tendencias. Tomo 2. 3a ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México - Instituto de Ciencias del Mar y Limnología, Centro de Investigación y de Estudios Avanzados - Unidad Mérida, Campeche, pp. 787–816.
Hermosilla-Gómez, Z. 2009. Desarrollo metodológico para la correcta evaluación del estado ecológico de las aguas costeras de la Comunidad Valenciana, en el ámbito de la Directiva Marco del Agua, utilizando la clorofila a como parámetro indicador de la calidad. Tesis de Doctorado. Universidad Politécnica de Valencia, Valencia. 156 pp.
Holl, C.M., T.A. Villareal, C.D. Payne, T.D. Clayton, C. Hart & J. Montoya. 2007. Trichodesmium in the western Gulf of Mexico: 15 N2-fixation and natural abundance stable isotope evidence. Limnology and Oceanography 52: 2249-2259. https://doi.org/10.4319/lo.2007.52.5.2249
Kelly-Gerreyn, B.A., T.R. Anderson, J.T. Holt, R.J. Gowen & R. Proctor. 2004. Phytoplankton community structure at contrasting sites in the Irish Sea: a modelling investigation. Estuarine, Coastal and Shelf Science 59: 363-383. https://doi.org/10.1016/j.ecss.2003.09.008
Komárek, J. & K. Anagnostidis. 1986a. Modern approach to the classification system of cyanophytes (Chroococcales). Archive für Hydrobiologie, Suppl. 43: 157-226.
Komárek, J. & K. Anagnostidis. 1986b. Modern approach to the classification system of cyanophytes (Nostocales). Archive für Hydrobiologie, Suppl. 56: 247-345.
Kottuparambil, S., P. Jin & S. Agusti. 2019. Adaptation of red sea phytoplankton to experimental warming increases their tolerance to toxic metal exposure. Frontiers in Environmental Science 7: 125. https://doi:10.3389/fenvs.2019.00125
Kurczyn, J.A., C.M. Appendini, E. Beier, A. Sosa-López, J. López-González, G. Posada-Vanegas. 2020. Oceanic and atmospheric impact of central American cold surges (Nortes) in the Gulf of Mexico. International Journal of Climatology 41: E1450-E1468. https://doi:10.1002/joc.6779
Kurczyn, J.A., R. Duran, E. Beier & A.J. Souza. 2021. On the advection of upwelled water on the western Yucatan Shelf. Frontiers in Marine Science 8: 723452. https://doi.org/10.3389/fmars.2021.723452
Landsberg, J.H. 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science and Aquaculture 10: 113-390. https://doi.org/10.1080/20026491051695
Lenes, J.M., B.P. Darrow, C. Cattrall, C.A. Heil, M. Callahan, G.A. Vargo, R.H. Byrne, J.M. Prospero, D.E. Bates, K.A. Fanning & J.J. Walsh. 2001. Iron fertilization and the Trichodesmium response on the West Florida shelf. Limnology and Oceanography 46: 1261-1277. https://doi.org/10.4319/lo.2001.46.6.1261
Livingston, R.J. 2001. Eutrophication processes in coastal systems: origin and succession of plankton blooms and effects on secondary production in Gulf Coast estuaries. Center for Aquatic Research and Resource Management, Florida State University, CRC Press, Boca Raton.
Masters, G. & W.P. Ela. 2008. Introduction to environmental engineering and science. 3rd ed. Prentice-Hall, Englewood Cliffs.
Merino-Virgilio, F.C., Y.B. Okolodkov, A.C. Aguilar-Trujillo & J.A. Herrera-Silveira. 2013. Phytoplankton of the northern coastal and shelf waters of the Yucatan Peninsula, southeastern Gulf of Mexico, Mexico. Check List 9: 771-779. https://doi.org/10.15560/9.4.771
Merino-Virgilio, F.C., Y.B. Okolodkov, A.C. Aguilar-Trujillo, I. Osorio-Moreno, I. Gárate-Lizárraga, L. Ector & J.A. Herrera-Silveira. 2019. Blooms caused by the diatom Cylindrotheca closterium along the northern coast of Yucatan, southeastern Gulf of Mexico (2001-2014). In: A. Kovács & P. Nagy. Eds. Advances in marine biology. Nova Science Publishers Inc., Hauppauge, pp. 52-72.
Monreal-Gómez, M.A., D.A. Salas de León & A.G. Gracia. 2004. Golfo de México: circulación y productividad. Ciencias 76: 24-33.
Monreal-Gómez, M.A., D.A. Salas de León, A.M. Padilla-Pilotze & M.A. Alatorre-Mendieta. 1992. Hidrografía y estimación de corrientes de densidad en el sur de la Bahía de Campeche, México. Ciencias Marinas 18: 115-133. http://dx.doi.org/10.7773/cm.v18i4.908
Muciño-Álvarez, R.E., M.G. Figueroa-Torres & A. Aguirre-León. 2015. Cianofitas de los sistemas fluvio-lagunares Pom-Atasta y Palizada del Este, adyacentes a la Laguna de Términos, Campeche, México. Polibotánica 39: 49-78.
Osorio-Tafall, B.F. 1942. Notas sobre algunos dinoflagelados planctónicos marinos de México, con descripción de nuevas especies. Anales de la Escuela Nacional de Ciencias Biológicas 2: 435-447.
Ospina-Álvarez, N. & E.J. Peña. 2004. Alternativas de monitoreo de calidad de aguas: algas como bioindicadores. Acta Nova 2: 513-517.
Páez-Osuna, F. 2005. Fuentes de metales en la zona costera marina. In: A.V. Botello, J. Rendón-von Osten, J.A. Benítez, G. Gold-Bouchot. Eds. Golfo de México. Contaminación e impacto ambiental: diagnóstico y tendencias Tomo 1. 3a ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México - Instituto de Ciencias del Mar y Limnología, Centro de Investigación y de Estudios Avanzados - Unidad Mérida, Campeche, pp. 329–342.
Poot-Delgado, C.A. 2016. Florecimientos algales nocivos en las costas de Campeche, Golfo de México. Investigación y Ciencia de la Universidad Autónoma de Aguascalientes 67: 91-96. https://doi.org/10.33064/iycuaa2016682264
Poot-Delgado, C.A. & Y.B. Okolodkov. 2016. Microalgae as water quality indicators: an overview. In: M. Snyder. Ed. Aquatic ecosystems influences, interactions and impact on the environment. Nova Science Publishers Inc., Hauppauge, pp. 41–65.
Poot-Delgado, C.A., P.I. Rosado-García & Y.A. Guzmán-Noz. 2014. Fitoplancton marino potencialmente nocivo en las aguas costeras de Campeche. In: A.V. Botello, J. Rendón-von Osten, J.A. Benítez, G. Gold-Bouchot. Eds. Golfo de México. Contaminación e impacto ambiental: diagnóstico y tendencias Tomo 1. 3a ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México-Instituto de Ciencias del Mar y Limnología, Centro de Investigación y de Estudios Avanzados - Unidad Mérida, Campeche, pp. 117–132.
Poot-Delgado, C.A., Y.B. Okolodkov & J. Rendón-von Osten. 2022. Spatio-temporal variation of harmful planktonic microalgae and cyanobacteria along the central coast of Campeche, southeastern Gulf of Mexico. Bulletin of Environmental Contamination and Toxicology 108: 15-23. https://doi.org/10.1007/s00128-021-03203-w
Poot-Delgado, C.A., Y.B. Okolodkov, J.A. Aké-Castillo & J. Rendón-von Osten. 2018. Potentially harmful cyanobacteria in oyster banks of Términos lagoon, southeastern Gulf of Mexico. Acta Biológica Colombiana 23: 51-58. http://dx.doi.org/10.15446/abc.v23n1.65809
Praveen-Kumar, M., T. Madhusudana-Reddy, P. Nithila & S. Jayarama-Reddy. 2005. Distribution of toxic trace metals Zn, Cd, Pb, and Cu in Tirupati soils, India. Soil and Sediment Contamination: An International Journal 14: 471-478. https://doi.org/10.1080/15320380500263667
Rendón von-Ostén, J. & M. Lara-Flores. 2015. Coliformes fecales y totales en agua de tres sistemas acuáticos de la zona costera de Campeche. In: E.F. Kauffer-Michel & D. Escobar-Castillejos. Eds. De Chiapas a la Península de Yucatán: intersticios hídricos. Universidad Autónoma de Chiapas, Tuxtla Gutiérrez, pp. 183–194.
Revilla, M., J. Franco, J. Bald, Á. Borja, A. Laza, S. Seoane & V. Valencia. 2009. Assessment of the phytoplankton ecological status in the Basque coast (northern Spain) according to the European Water Framework Directive. Journal of Sea Research 61: 60–67. https://doi.org/10.1016/j.seares.2008.05.009
Saito, M.A., J.G. Tyler & J.T. Ritt. 2008. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnology and Oceanography 53: 276-290. https://doi.org/10.4319/lo.2008.53.1.0276
Schiller, J. 1931. Dinoflagellatae (Peridineae) in monographischer Behandlung. 1. Teil, Lieferung 1. In: Kolkwitz, R., Zehnter Band. Flagellatae. Dr. L. Rabenhorst’s Kryptogammen-Flora von Deutschland, Österreich und der Schweiz. Akademische Verlagsgesellschaft, Leipzig, Germany, pp. 1-256.
SE (Secretaría de Economía). 2016. Norma Mexicana NMX-AA-120-SCFI-2016. Que establece los requisitos y especificaciones de sustentabilidad de calidad de playas. Secretaria de Economía, Diario Oficial de la Federación, 7 de diciembre de 2016. México.
SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). 2014. El medio ambiente en México 2013-2014. https://apps1.semarnat.gob.mx:8443/dgeia/informe_resumen14/00_mensajes/00_intro.html
SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). 1996. Norma Oficial Mexicana NOM-001-ECOL-1996. Que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. Secretaria de Medio Ambiente, Recursos Naturales y Pesca, Diario Oficial de la Federación, 6 de enero de 1997. México, D.F., México.
SSA (Secretaría de Salud). 1994. Norma Oficial Mexicana NOM-112-SSA1-1994. Bienes y servicios. Determinación de bacterias coliformes. Técnica del número más probable. Secretaría de Salud, Diario Oficial de la Federación, 19 de octubre de 1995. México, D.F., México.
Talarmin, A., F. Van-Wambeke, P. Lebaron & T. Moutin. 2015. Vertical partitioning of phosphate uptake among picoplankton groups in the low Pi Mediterranean Sea. Biogeosciences 12: 1237-1247.
Ter Braak, C.J.F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167-1179. https://doi.org/10.2307/1938672
Throndsen, J. 1978. Preservation and storage. In: A. Sournia. Ed. Phytoplankton manual. UNESCO Monographs on Oceanographic Methodology 6, United Nations Education, Science and Culture Organization, Paris, pp. 69–74.
UNEP (United Nations Environment Programme). 1991. Standard chemical methods for marine environmental monitoring. Reference Methods for Marine Pollution Studies 50. United Nations Environment Program, Kenya.
USEPA (United States Environmental Protection Agency). 1996. Method 3050B (SW-846): acid digestion of sediments, sludges, and soils. Test methods for evaluating soil waste SW-846. Environmental Protection Agency, Cincinnati.
Utermöhl, H. 1958. Zur vervolkommung der quantitative. Phytoplankton-Methodik. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9: 1-38.
Varona-Cordero, F., F.J. Gutiérrez-Mendieta, A.Z. Márquez-García, A.B. Crevenna-Recásens & V. Torres-Rodríguez. 2014. Variación espacio-temporal de las características físico-químicas y nutrientes en la región marino-costera ubicada entre los ríos San Pedro y San Pablo y Champotón, Campeche. In: A.V. Botello, J. Rendón-von Osten, J.A. Benítez & G. Gold-Bouchot. Ed. Golfo de México. Contaminación e impacto ambiental: diagnóstico y tendencias. Tomo 2. 3a ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México - Instituto de Ciencias del Mar y Limnología, Centro de Investigación y de Estudios Avanzados - Unidad Mérida, Campeche, pp. 839-866.
Vázquez-Gutiérrez, F., H. Dorantes-Velázquez, H. Alexander-Valdés & A. Frausto-Castillo. 1988. Estudio hidrológico de las aguas costeras, frente a las bocas de la laguna de Términos, Campeche, en dos épocas climáticas diferentes. Anales del Instituto de Ciencias del Mar y Limnología – Universidad Nacional Autónoma de México 5: 183-194.
Villanueva, F.S. & A.V. Botello. 1998. Metal pollution in coastal areas of Mexico. Reviews of environmental contamination and toxicology. Reviews of Environmental Contamination and Toxicology 157: 53-94. https://doi.org/10.1007/978-1-4612-0625-5_3
White, A.E., F.G. Prahl, R.M. Letelier & B.N. Popp. 2007. Summer surface waters in the Gulf of California: prime habitat for biological N2 fixation. Global Biogeochemical Cycles 21: 1-11. https://doi.org/10.1029/2006GB002779
Yáñez-Arancibia, A. & J.W. Day. 2005. Ecosystem functioning: the basis for sustainable management of Términos Lagoon, Campeche, México. Instituto de Ecología A.C., Jalapa, Veracruz, Mexico. 76 pp.
Zavala-Hidalgo, J., S.L. Morey & J.J. O’Brien. 2003. Seasonal circulation on the western shelf of the Gulf of Mexico using a high-resolution numerical model. Journal of Geophysical Research 108: C12. https://doi:10.1029/2003jc001879
Sometido: 13 mayo 2022
Revisado: 26 de junio 2022 (tres revisores anónimos)
Corregido: 30 de junio 2022
Aceptado: 30 de junio 2022