Spatial and temporal variation in the biomarkers of oxidative stress in red macroalgae Gracilaria vermiculophylla (Gracilariales, Gracilariaceae)
Variación espacio-temporal en los biomarcadores de estrés oxidativo en la macroalga roja Gracilaria vermiculophylla (Gracilariales, Gracilariaceae)
Paola A. Tenorio-Rodríguez1, Elisa Serviere-Zaragoza2, Lia C. Méndez-Rodriguez1, and Tania Zenteno-Savín1*
1Planeación Ambiental y Conservación, 2Ecología Pesquera, Centro de Investigaciones Biológicas del Noroeste, S.C., Calle Instituto Politécnico Nacional 195, Playa Palo de Santa Rita Sur, La Paz, Baja California Sur, CP 23096, México
*Corresponding author: email: tzenteno04@cibnor.mx
Tenorio-Rodríguez, P., E. Serviere-Zaragoza, L. C. Méndez-Rodriguez, & T. Zenteno-Savín . 2018. Spatial and temporal variation in the biomarkers of oxidative stress in red macroalgae Gracilaria vermiculophylla (Gracilariales, Gracilariaceae). Cymbella 4 (2-3): 57-68. http://cymbella.mx
Abstract
Macroalgae may be exposed to spatial and seasonal variations in environmental factors, such as irradiance (visible and ultraviolet radiation, UVR), temperature, salinity and exposure to air. Changes in any of these factors lead to increased reactive oxygen species (ROS) production and potential oxidative stress. Oxidative stress biomarkers were measured in the marine red algae Gracilaria vermiculophylla in the Baja California peninsula to assess effects of spatial and seasonal variability. Thiobarbituric acid reactive substances (TBARS) and carbonyl proteins levels were measured as markers of oxidative damage to lipids and proteins, respectively, in tallus samples. Polyphenols content and activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST) and glutathione reductase (GR) were quantified as antioxidant defenses. Polyphenols content, and activities of SOD, GPx and GST were higher in the warm season compared to the cold season. Antioxidant enzyme activities varied with site, being lower in “La Boca”, the deepest site, than in “La Estufa”, the shallower site. Higher antioxidant enzyme activities suggest an effective protection against ROS in the shallower regions, which may contribute to the ecological success of G. vermiculophylla along its vertical distribution, and may allow for adequate responses to the changing environmental conditions across the water column.
Keywords: Antioxidants, biomarkers, Gracilaria vermiculophylla, macroalgae, oxidative stress.Resumen
Las macroalgas pueden estar expuestas a variaciones espaciales y estacionales de los factores ambientales, como la radiación visible y la ultravioleta (UVR), la temperatura, la salinidad y la exposición al aire. Los cambios en estos factores conducen a aumentos en la producción de especies reactivas de oxígeno (ERO) y potencial estrés oxidativo. Los biomarcadores de estrés oxidativo se cuantificaron en la macroalga roja marina Gracilaria vermiculophylla en la península de Baja California para evaluar los efectos por la variabilidad espacio-temporal. Los niveles de sustancias reactivas al ácido tiobarbitúrico (TBARS) y a carbonilos protéicos se midieron como marcadores de daño oxidativo a lípidos y proteínas, respectivamente, en el talo. El contenido de polifenoles y la actividad de la superóxido dismutasa (SOD), la glutatión peroxidasa (GPx), la glutatión S-transferasa (GST) y la glutatión reductasa (GR) se cuantificaron como defensas antioxidantes. El contenido de polifenoles y actividades de SOD, GPx y GST fueron mayores en la estación cálida que en la fría. La actividad de las enzimas antioxidantes varió con el sitio; fue menor en el sitio ”La Boca”, en las muestras más profundas, en comparación a las muestras más someras del sitio “La Estufa”. Mayores actividades de enzimas antioxidantes sugieren protección efectiva contra ERO en las regiones someras, contribuyendo al éxito ecológio de G. vermiculophylla en su distribución vertical y permitiendo respuestas adecuadas a condiciones ambientales cambiantes de la columna de agua.
Key words: Palabras clave: antioxidantes, biomarcadores, Gracilaria vermiculophylla, macroalgas, estrés oxidativo.Introduction.
Gracilaria vermiculophylla (Ohmi) Papenfuss is a species of Northwestern Pacific origin, which is an invader in the east Pacific (Bellorin et al. 2004), inhabiting intertidal and subtidal zones, and may be found throughout the year (Thomsen & McGlathery 2007). The success of such cosmopolitan invader species has been related to traits that include regenerative abilities and capacity to survive to changing environmental factors (Nyberg & Wallentinus 2005).
In Northwestern Mexico, G. vermiculophylla has been present since at least 1979 (Bellorin et al. 2004), reflecting the first time a specimen was collected, rather than the date at which the introduction occurred. Its distribution and taxonomic confirmation in the region is analyzed by Krueger-Hadfield et al. (2016). In Mexico according with the Ponderación de Invasividad de Especies Exóticas, the species is classified as a high risk invasive species which, due to its characteristics and stress resistance, can thrive in diverse environments (SEMARNAT, 2017). Studying the antioxidant capacity and adaptive mechanisms in G. vermiculophylla may allow for understanding the features that allow certain species of macroalgae to thrive under various, seemingly extreme, conditions.
G. vermiculophylla occurs from shallow to deep water forming a vertical distribution of organisms along environmental gradients. This vertical zonation (defined as the vertical distribution of organisms, species, ecosystems (Benson 2002)) can be influenced by unpredictable factors, such as climate disruptions (e.g. storms), biological interactions (e.g. grazing, shading; Dayton 1975), and differential stress tolerance along the water column (e.g. desiccation, radiation), where a strong environmental stress gradient occurs perpendicular to the shore with the most extreme values towards the upper limit of the littoral zone (Chappuis et al. 2014; Davison & Pearson 1996).
Macroalgal zonation patterns have been related to the ability to resist a variety of potential stressful environmental conditions, including high radiation (visible and ultraviolet radiation, UVR), high and low temperature, desiccation, and osmotic stress (Davison & Pearson 1996; Flores-Molina et al. 2014; Lesser 2006; Phooprong et al. 2007). These factors may disrupt respiratory or photosynthetic metabolism, leading to the production of reactive oxygen species (ROS), including superoxide radical (O2•-), hydroxyl radical (HO•), and hydrogen peroxide (H2O2) (Collén & Davison 1999; Dring 2005; Lesser 2006). Oxidative stress, with a concomitant oxidative damage to cells and tissues, results from ROS production exceeding the antioxidant capacity (Halliwell & Gutteridge 2007). As other aerobic organisms, macroalgae may respond to an oxidative stress event by the activation of the antioxidant defense system, which includes enzymes and low-molecular-weight molecules. Superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx) and glutathione S-transferase (GST) are the main antioxidant enzymes (Halliwell & Gutteridge 2007). Polyphenols, ascorbate, chlorophylls, glutathione are among the non-enzymatic antioxidants distributed in higher plants and algae (Abdala-Díaz et al. 2014; Celis-Plá et al. 2014; Flores-Molina et al. 2014). Studies related to the scavenging mechanisms for protection against oxidative damage in macroalgae are scarce; however, evidence suggests a correlation between antioxidant capacity and tolerance to environmental stressors in higher plants (Peltzer & Polle 2001; Tian & Yu 2009), green and brown algae (Aguilera et al. 2002; Choo et al. 2004; Dring 2005; Flores-Molina et al. 2014), as well as some red algae (Burritt et al. 2002; Kumar et al. 2010; Maharana et al. 2015; Pise et al. 2013).
Enzyme, in particular SOD, activities in different algal groups have been related to a species’ vertical distribution and tolerance to solar radiation exposure (Aguilera et al. 2002; Choo et al. 2004; Dring 2005). SOD scavenges O2-, the initiator of the ROS production and oxidative damage cascades. GPx and GR play important roles in plant responses to environmental stressors, including changes in temperature (Aguilera et al. 2002; Betancor et al. 2015; Choo et al. 2004;). Another antioxidant defense mechanism against solar radiation in plants are the phenolic compounds (Aguilera et al. 2002); these substances can act as photoprotector agents against intense solar irradiance by absorbing incident photons, or indirectly by transferring hydrogen atoms to lipid peroxyl radicals (Abdala-Díaz et al. 2014; Tenorio-Rodríguez et al. 2017). ROS produced in chloroplasts can interact with many biomolecules inducing the formation of fatty acid hydroperoxides and oxidation of proteins (Choo et al. 2004).
Ecophysiological studies of macroalgae suggest a strong relationship of the antioxidant capacity with algal zonation patterns, as well as tolerance to desiccation, temperature, and irradiation, particularly for some brown and red macroalgae species, such as Fucus spp., Chondrus crispus Stackhouse (Collén & Davison 1999), and Bostrychia arbuscula W.H. Harvey (= Stictosiphonia arbuscula) (Burrit et al. 2002; Dring 2005). Contreras-Porcia et al. (2011) suggest that Pyropia columbina (Montagne) W.A. Nelson (= Porphyra columbina) exposed to natural desiccation during low tide has elevated activities of antioxidant enzymes and high concentration of photosynthetic pigments. This does not seem to be the case for species that inhabit the lower intertidal zone (Flores-Molina et al. 2014).
The objective of this study was to examine the changes in antioxidant enzyme activities, polyphenol content and oxidative damage of G. vermiculophylla at different sites, to reflect the vertical distribution of this species, and seasons. The results contribute to the identification of physiological strategies employed by G. vermiculophylla to cope with environmental stressors associated with zonation and seasonal changes.
MATERIAL AND METHODS
Sampling was performed in Estero Banderitas, situated at 24° 15’ - 25° 20’ N and 112° 15’ W within the Bahía Magdalena – Almejas complex, Baja California, Mexico. This is a coastal lagoon complex, where the sea surface water temperature ranges from 18 to 31 ºC in winter and summer, respectively (Álvarez-Borrego et al. 1975). The sampling area is considered pristine and unpolluted (Escobar-Sánchez et al. 2011). G. vermiculophylla species were collected by scuba diving in three sampling sites: “La Estufa”, mean depth 0.5-1 m, “El Conchalito”, mean depth 7 m, and “La Boca”, mean depth 20 m according to its distribution at different depths along the estuary in November 2009, February, April and June 2010 (Fig. 1). During each visit, healthy fronds of G. vermiculophylla were randomly collected. Macroalgae thallus were cleaned by hand to remove epiphytes, pooled, and randomly separated in five replicates of 5-10 individuals each at each site. Samples were dried, milled and frozen by inmersion in liquid nitrogen.
Prior to SOD, GR, GPx and GST enzymatic activity determinations, tallus samples (0.2 g fresh weight) of G. vermiculophylla were ground in liquid nitrogen with potassium phosphate buffer (50 mM, pH 7.0) containing 0.25 % (v/v) Triton X-100 (w/v), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 % polyvinylpyrrolidone (PVPP). Extracts were centrifuged at 15,000 g for 10 min at 4 °C before assaying. In order to standardize the results for enzyme activities, protein concentration in the extracts was determined on a microplate reader (Multiscan FC Thermo Fisher, Vantaa, Finland) using the method described by Bradford (1976) with bovine serum albumin (BSA) as a standard (Bio-Rad Laboratories, Hercules, CA., USA).
Superoxide dismutase (SOD): The activity of SOD was assayed following the inhibition of the reduction of nitroblue tetrazolium (NBT) by O2•-, yielding formazan, at 560 nm according to Suzuki (2000). SOD activity is expressed in units (U) mg-1 of protein. One unit of SOD activity is defined as the amount of enzyme needed to inhibit the maximum reaction by 50 %.
Glutathione S-transferase (GST): GST activity was measured at 340 nm following the formation of tioether glutathione dinitrobencene as a product of the reaction between the tripeptide glutathione (GSH) and 1-chloro-2,4-dinitrobencene (Habig & Jakoby 1981). GST activity is expressed in U mg-1 of protein. One unit of GST activity is defined as the amount of enzyme that synthesizes 1 μmol of product min-1 .
Glutathione peroxidase (GPx): GPx activity was measured by monitoring the continuous decrease in reduced nicotinamide adenine dinucleotide phosphate (NADPH) concentration using H2O2 as a substrate at 340 nm (Folhé & Günzler 1984). One unit of GPx activity is defined as the amount of enzyme that oxidizes 1 μmol of NADPH min-1 . GPx activity is expressed in U mg-1 of protein.
Glutahione reductase (GR): GR activity was measured at 340 nm monitoring the oxidation of NADPH by oxidized glutathione (GSSG) (Goldberg & Spooner 1987). One unit of GR activity is defined as the amount of enzyme that reduces 1 μmol of GSSG min-1 . GR activity is expressed in U mg-1 of protein.
The total concentration of polyphenols was quantified using the Folin-Ciocalteu colorimetric method (Singleton & Rossi 1965). In brief, 1 g of fresh sample was homogenized with a mixture of water:methanol:acetone (2:3:5 v/v). Samples were incubated in a water bath at 65 °C with agitation for 1 h. Sodium carbonate (Na2CO3) was added and samples were incubated for 1 h at room temperature. The absorbance at 750 nm was recorded in a microplate reader (BioRad TM 550, Hercules, CA, USA) and compared to a gallic acid calibration curve. The results are expressed as gallic acid equivalents (GAE) in mg g-1 fresh weight (f.w.).
The levels of lipid peroxidation were determined as the thiobarbituric acid reactive substances (TBARS) content (Persky et al. 2000), as previously described (Labrada-Martagón et al. 2011; López-Cruz et al. 2010). Results were expressed in nmoles of TBARS mg-1 of wet tissue.
Oxidative damage to proteins was assessed as the content of protein carbonyls (Levine et al. 1994). Extracts were incubated with 10 mM 2,4-dinitrophenyl-hidrazine for 60 minutes at ambient temperature. Proteins were precipitated with trichloroacetic acid; the pellet formed after centrifugation was washed twice with ethanol:ethyl acetate (1:1) and dissolved in 6 M guanidine. The protein carbonyl content was determined spectrophotometrically at 370 nm. Results were expressed as μmoles of protein carbonyls mg-1 of tissue.
STATISTICAL ANALYSIS
Shapiro-Wilks test was used to test for normality and Bartlett´s test to determine the homoscedasticity of variance of the variables (Zar 1999). Data were natural log (ln) transformed prior to running the parametric analyses. In order to evaluate seasonal effects, data were grouped as cold (February and April; ~18 °C) or warm (November and June; ~31 °C) season according to the physicochemical characteristics in Bahía Magdalena (Koch et al. 2007), and were analyzed by two-factor ANOVA with site and season as factors and oxidative stress biomarkers as dependent variables. Tukey’s post hoc analysis was used when differences were detected. Statistical significance was set at p<0.05. All statistical analyses were performed using GraphPad PRISM® software 5.0 (Statsoft, Tulsa, OK).
RESULTS
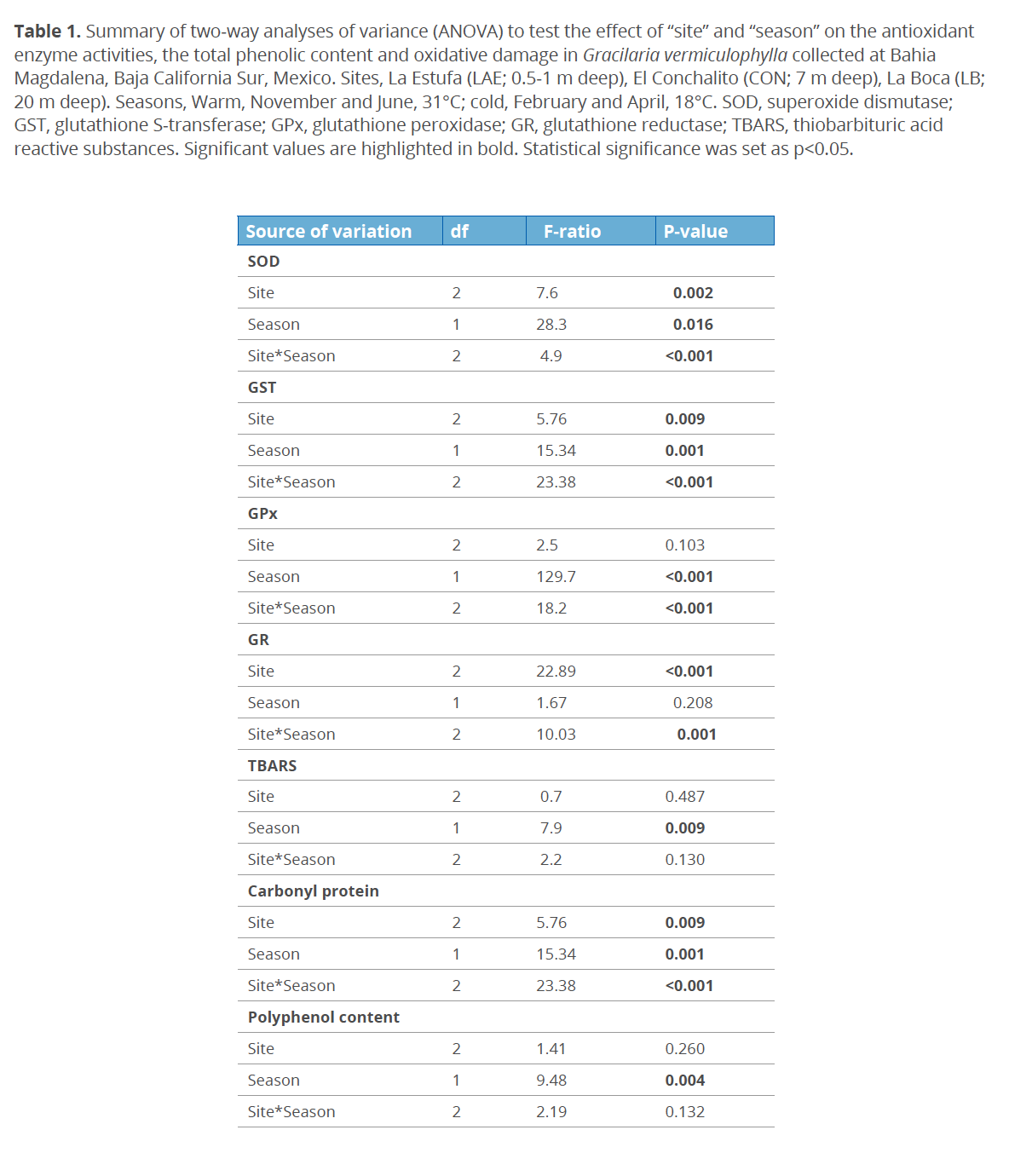
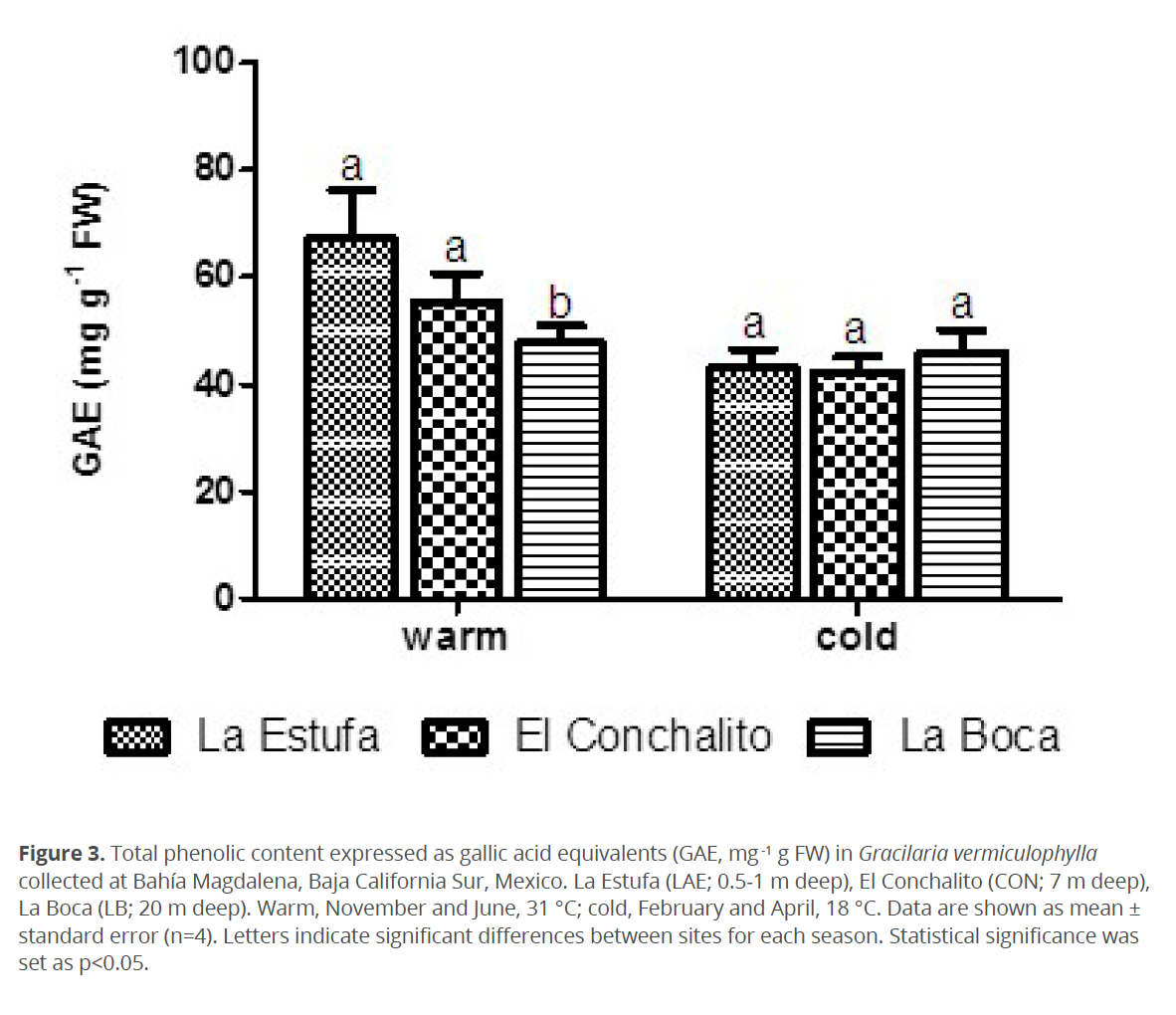
To investigate the spatial and seasonal changes in the protection mechanism against oxidative stress in G. vermiculophylla, the activity of antioxidant enzymes and polyphenols content were quantified. Results for the activity of antioxidant enzymes and polyphenols content are shown in figures 2 and 3, respectively. Significant differences in the antioxidant enzyme activities were found between sampling sites and seasons. SOD activity was higher in “La Estufa”, the shallower (0.5-1 m deep) site, and lower in “La Boca”, the deepest (20 m deep) site (p<0.05). The SOD activity in G. vermiculophylla was higher in the warm season (November and June; ~31 °C) compared to the cold season (February and April; ~18 °C) (p<0.05) (Table 1). During the warm season, but not during the cold season, significant differences in SOD activity by site were observed (Fig. 2).
Significantly higher GST activity was observed in “La Estufa” during the warm season compared with the other sampled sites (p<0.05) (Fig. 2). Similarly GPx activity in G. vermiculophylla from “La Estufa” was higher in comparison with the other sites (p<0.05) (Fig. 2). In “La Estufa”, GPx activity was lower in the cold season compared with the warm season (p<0.05) (Table 1). There was no significant difference in the activity of GR neither between sites nor between seasons (p>0.05).
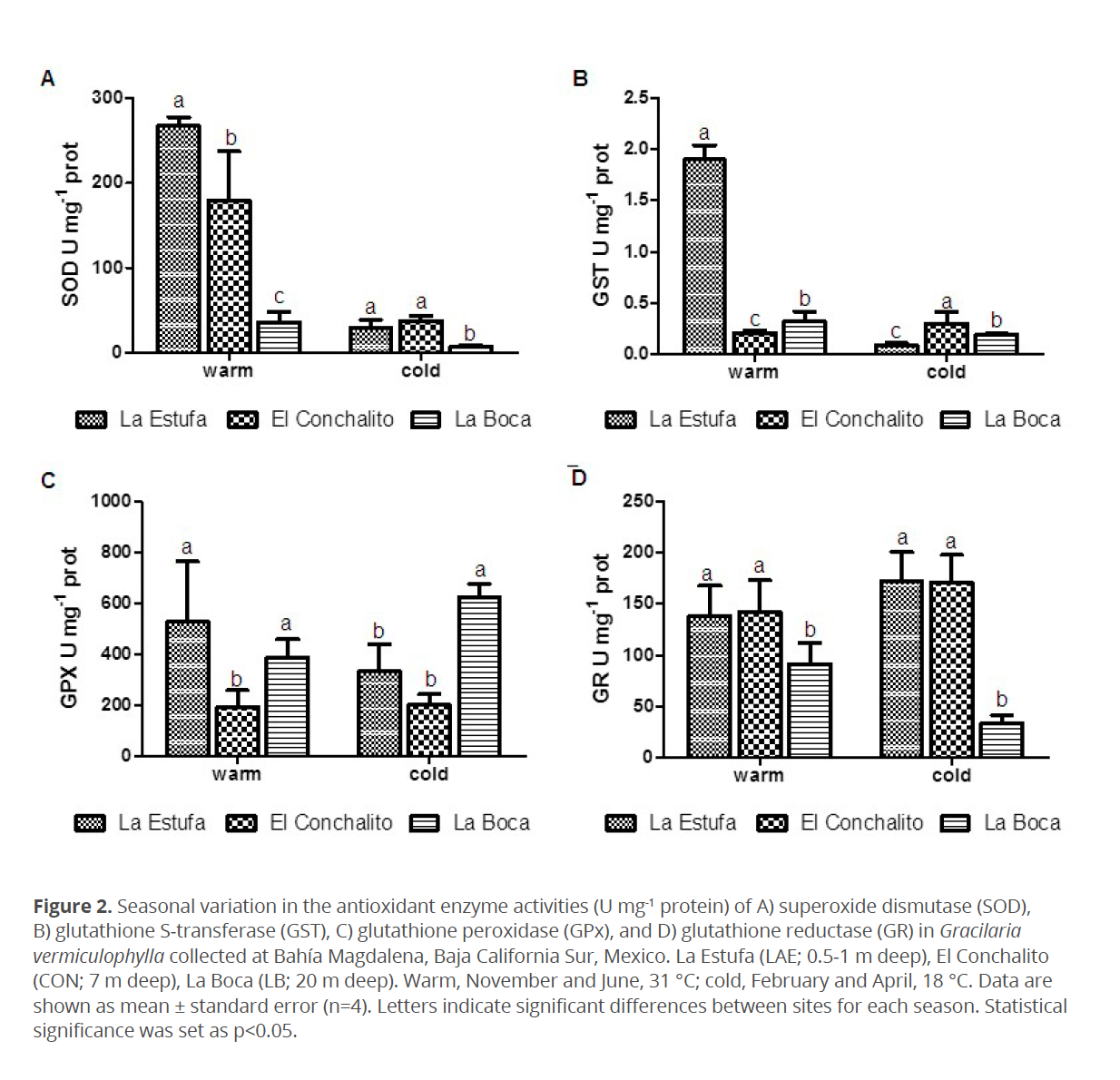
The total phenolic content of G. vermiculophylla was higher in “La Estufa”, the shallower (0.5-1 m) site, and in “El Conchalito” (mid-depth; ~7 m) than “La Boca” (deepest site; 20 m) (p<0.05) (Fig. 3) (Table 1) during the warm season. The phenolic content was lower in the three sites during the cold season, and no differences between sites were found in the cold season (Fig. 3).
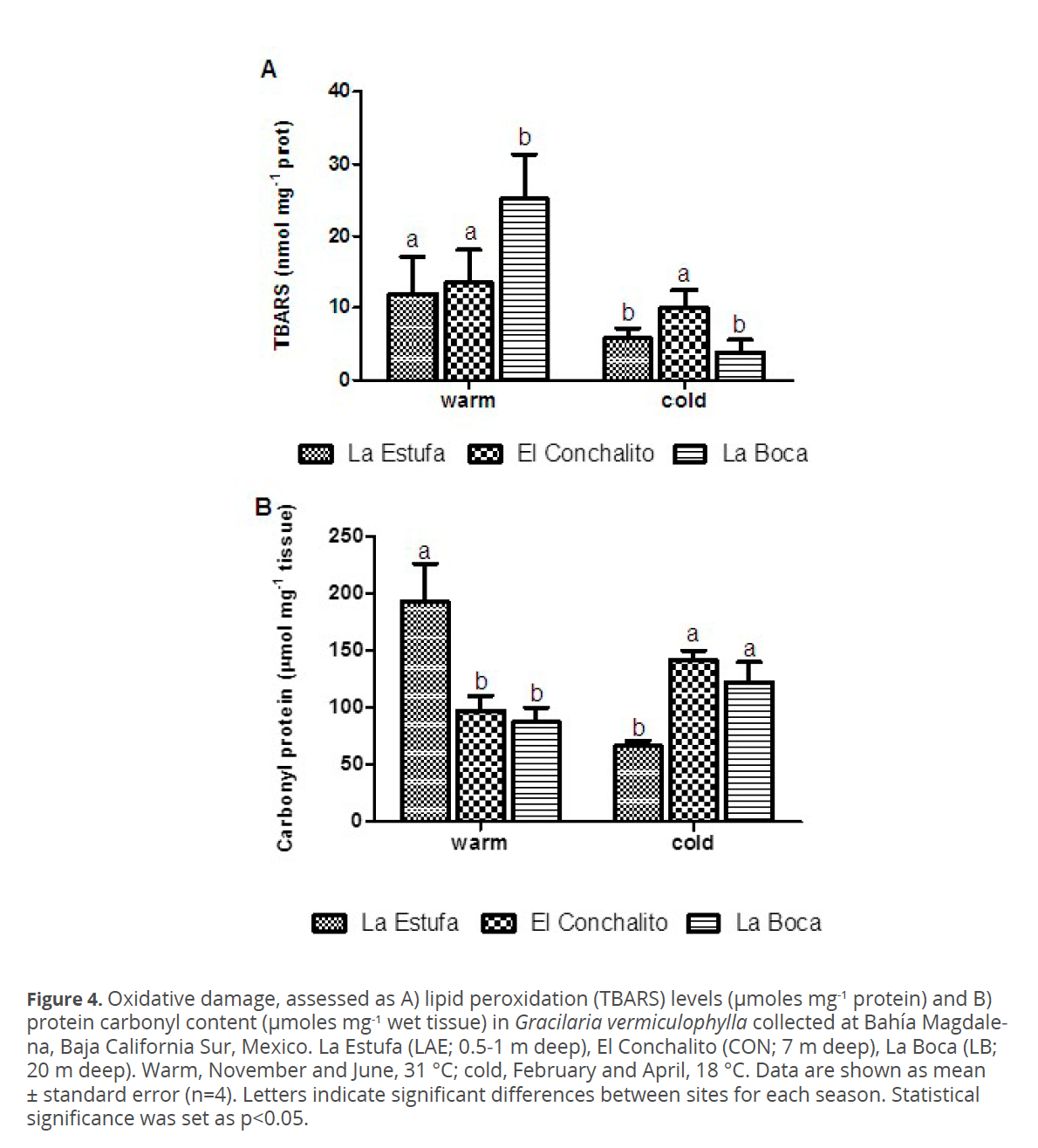
To investigate the spatial and seasonal changes in oxidative damage to lipids and proteins in G. vermiculophylla, content of TBARS and protein carbonyls were quantified. Results of oxidative damage are shown in figure 4. No significant differences in TBARS levels were found between sites in either season (p>0.05) (Table 1). TBARS levels were significantly higher in the warm season compared to cold season within each site (p<0.05) (Fig. 4).
Significant differences in the level of protein carbonyls were found between sites and among seasons (Fig. 4). In “La Estufa” during the warm season protein carbonyl levels were higher than those recorded in the same site in the cold season (p<0.05).
DISCUSSION
In nature, algae are not exposed to factors independently, but collectively experience stressors that may have synergestic effects. In Estero Banderitas, G. vermiculophylla showed higher SOD activity in the shallower site, “La Estufa”, during the warm (November and June; 31 °C) season, it appears that SOD activity is dependent on depth and season. Similar results have been reported for the red algae Devaleraea ramentacea (Linnaeus) Guiry and Palmaria palmata (Linnaeus) Weber & Mohr, which typically occur in the upper sublittoral zone (Aguilera et al. 2002). Penetration of the solar radiation into the water column is attenuated with increasing water depth; therefore, lower antioxidant defenses are expected in algae inhabiting the deepest water layers, as was observed in this study.
In this study, G. vermiculophylla exhibited spatial and temporal differences in the activities of SOD, GPx and GST. It is possible that during the summer months when temperatures, solar irradiation, as well desiccation conditions are at their peak, the interaction of these factors trigger an increase in antioxidant defenses in this species in contrast with the cold season. Similar results in the activities of these enzymes have been reported for other algae species exposed to abiotic stresses which induce overproduction of ROS (Contreras-Porcia et al. 2011; Flores-Molina et al. 2014; Kumar et al. 2010). In red algae Mastocarpus stellatus (Stackhouse) Guiry and Chondrus crispus Stackhouse and in green algae Ulva pseudorotundata Cormaci, Furnari & Alongi (= Ulva rotundata), the efficiency of ROS scavenging was partly related to the species’ zonation pattern along its vertical distribution and the radiation conditions (Bischof et al. 2003; Collén & Davison 1999). Similarly, Maharana et al. (2015) reported increased antioxidant activities as well photosynthetic pigments in the red algae Hypnea musciformis (Wulfen) Lamouroux during summer months.
The phenolic content in G. vermiculophylla in this study was 78 % higher at the shallow site during the warm season, and 1 % lower in the deeper site. Similar observations were reported by Abdala-Díaz et al. (2006) and Betancor et al. (2015) for brown algae Cystoseira tamariscifolia (Hudson) Papenfuss, C. humilis Schousboe ex Kützing and red algae Digenea simplex (Wulfen) C. Agardh. The difference between seasons in the polyphenol content could be related to the lower irradiance exposure and photosynthetically active radiation, as it has been reported previously for brown algae C. tamariscifolia and Desmarestia anceps Montagne and for red algae Chondrus crispus and Mastocarpus stellatus (Celis-Plá et al. 2014; García-Sánchez et al. 2014; Flores-Molina et al. 2016; Lohrmann et al. 2004). Increased content of phenolic compounds is a complementary strategy to increased activity of antioxidant enzymes in avoidance of oxidative damage.
Low lipid peroxidation and high protein carbonyl levels found in G. vermiculophylla in the shallow site (“La Estufa”) during the warm season resulted quite intriguing. In this context, the low lipid peroxidation may be related to the activities of key antioxidant enzymes SOD and GST. During events that potentially lead to oxidative stress (e.g., excessive or prolonged radiation, temperature, or a combination of these factors, as observed during the warm season), the antioxidant system is finely tuned to respond accordingly, and contribute to avoidance of oxidative damage. However, the observed levels of protein carbonyls in G. vermiculophylla suggest the involvement of other pathways in the oxidative stress-mediated induction of cell injury, as proposed for higher plants (Anjum et al. 2015; Boscolo et al. 2003). The combined results from this study suggest that the antioxidant defenses may contribute to the ecological success of G. vermiculophylla along its vertical distribution, and may allow for adequate responses to the changing environmental conditions along the water column.
CONCLUSION
It was found that the antioxidant enzyme activity and the polyphenol content in G. vermiculophylla are higher in the shallower site (“La Estufa”, 0.5-1 m deep), where this species is exposed to drastic changes in environmental conditions, especially during the warm season (November and June, 31 °C). G. vermiculophylla seems to be a stress-tolerant species in which the antioxidant defense systems, including the antioxidant enzymes and polyphenols, contribute to protection against ROS; thus, allowing this species to cope with changing environmental conditions.
ACKNOWLEDGEMENTS
Authors appreciate the assistance of H. Bervera León and J. Angulo Calvillo in the field. N.O. Olguin-Monroy and O. Lugo-Lugo at Laboratorio de Estrés Oxidativo, as well as A. Mazariegos and students at Laboratorio de Macroalgas (CIBNOR) in sample collection, processing and technical assistance. This study was funded by Centro de Investigaciones Biológicas del Noroeste S.C. (CIBNOR, PC2.0, PC0.10).
REFERENCES
Abdala-Díaz, R.T., A. Cabello-Pasini, E. Pérez-Rodríguez, R.M. Conde-Álvarez & F.L. Figueroa. 2006. Daily and seasonal variations of optimum quantum yieldand phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Marine Biology 148: 459-465.
Abdala-Díaz, R.T., A. Cabello-Pasini, E. Márquez-Garrido & F. López Figueroa. 2014. Intra-thallus variation of phenolic compounds, antioxidant activity, and phenolsulphatase activity in Cystoseira tamariscifolia (Phaeophyceae) from southern Spain. Ciencias Marinas 40: 1-10.
Aguilera, J., A. Dummermuth, U. Karsten, R. Schriek & C. Wiencke. 2002. Enzymatic defences against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biology 25: 432-441.
Álvarez-Borrego, S., B.P. Flores-Báez & L.A. Galindo-Bect. 1975. Hidrología del Alto Golfo de California II. Condiciones durante Invierno, Primavera y Verano. Ciencias Marinas 2: 21-36.
Anjum, N.A., A. Sofo, A. Scopa, A. Roychoudhury, S.S. Gill, M. Iqbal, A.S. Lukatkin, E. Pereira, A.C. Duarte & I. Ahmad. 2015. Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research 22: 4099-4121.
Bellorin, A.M., M.C. Oliveira & E.C. Oliveira. 2004. Gracilaria vermiculophylla: A western Pacific species of Gracilariaceae (Rhodophyta) first recorded from the eastern Pacific. Phycological Research 52: 69-79.
Benson, K.R. 2002. The study of vertical zonation on rocky intertidal shores—a historical perspective. Integrative and Comparative Biology 42: 776-779.
Betancor, S., B., Dominguez, F. Tuya, F.L. Figueroa & R. Haroun. 2015. Photosynthetic perfomance and photoprotection of Cystoseira humilis (Phaeophyceae) and Digenea simplex (Rhodophyceae) in an intertidal rock pool. Aquatic Botany 121: 16-25.
Bischof, K., P.J. Janknegt, A.G.J. Buma, J.W. Rijstenbil, G. Peralta & A.M. Breeman. 2003. Oxidative stress and enzymatic scavenging of superoxide radicals induced by solar UV-B radiation in Ulva canopies from southern Spain. Scientia Marina 67: 353-359.
Boscolo, P.R.S., M. Menossi & R.A. Jorge. 2003. Aluminum-induced oxidative stress in maize. Phytochemistry 62: 181-189.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254.
Burritt, D.J., J. Larkindale & K.L. Hurd. 2002. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 215: 829-838.
Celis-Plá, P.S.M., N. Korbee, A. Gómez-Garreta & F.L. Figueroa. 2014. Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Scientia Marina 78: 377-388.
Chappuis, E., M. Terradas, M.E. Cefalì, S. Mariani & E. Ballesteros. 2014. Vertical zonation is the main distribution pattern of littoral assemblages on rocky shores at a regional scale. Estuarine, Coastal and Shelf Science 147: 113-122.
Choo, K.S., P. Snoeijs & M. Pedersen. 2004. Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. Journal of Experimental Marine Biology and Ecology 298: 111-123.
Collén, J. & I.R. Davison. 1999. Stress tolerance and reactive oxygen metabolism in the intertidal seaweeds Mastocarpus stellatus and Chondrus crispus. Plant Cell & Environment 22: 1143-1151.
Contreras-Porcia, L., D. Thomas, V. Flores & J.A. Correa. 2011. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). Journal of Experimental Botany 62: 1815-1829.
Davison, I.R. & G.A. Pearson. 1996. Stress tolerance in intertidal seaweeds. Journal of Phycology 32: 197-211.
Dayton. P.K. 1975. Experimental evaluation of ecological dominance in a rocky intertidal algal community. Ecological Monographs 45: 137-159.
Dring, M.J. 2005. Stress resistance and disease resistance in seaweeds: the role of reactive oxygen metabolism. In: J.A. Callow. Ed. Advances in Botanical Research 43. Incorporating Advances in Plant Pathology. Academic Press, New York, pp. 175-207
Escobar-Sánchez, O., F. Galván-Magaña & R. Rosíles-Martínez. 2011. Biomagnification of mercury and selenium in blue shark Prionace glauca from the Pacific Ocean of Mexico. Biological Trace Element Research 144: 550-559.
Flores-Molina, M.R., D. Thomas, C. Lovazzano, A. Núñez, J. Zapata, M. Kumar & L. Contreras-Porcia. 2014. Desiccation stress in intertidal seaweeds: Effects on morphology, antioxidant responses and photosynthetic performance. Aquatic Botany 113: 90-99.
Flores-Molina, M.R., R. Rautenberger, P. Muñoz, P. Huovinen & I. Gómez. 2016.Stress tolerance of the endemic antarctic brown alga Desmarestia anceps to UV radiation and temperature is mediated by high concentrations of phlorotannins. Photochemical & Photobiological Sciences 92: 455-466.
Folhé, L. & W.A. Günzle. 1984. Assays of glutathione peroxidase. Methods in Enzymology 105: 114-120.
García-Sánchez, M., N. Korbee, I.M. Pérez-Ruzafa, C. Marco, F. López-Figueroa & A. Pérez-Ruzafa. 2014. Living in a coastal lagoon environment: Photosynthetic and biochemical mechanisms of key marine macroalgae. Marine Environmental Research 101: 8-21.
D. M. Goldberg and R. J. Spooner, “Glutathione Reductase,” In: H. U. Bergmeyer, J. Bergmeyer and M. GraBI, Eds., Methods of Enzymatic Analysis, 3rd Edition, Verlag Chemie, Weinheim, 1983, pp. 258-265..
Habig, W.H. & W.B. Jakoby. 1981. Glutathione S-transferases (rat and human). Methods in Enzymology 77 Academic Press, New York, pp 218-235.
Halliwell, B. & J.M.C. Gutteridge. 2007. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press, New York.
Koch, V., L.B. Brooks, & W.J. Nichols. 2007. Population ecology of the green/black turtle (Chelonia mydas) in Bahía Magdalena, Mexico. Marine Biology 153: 35-46.
Krueger-Hadfield, S.A., G. Hernández Carmona, R. Terada, J.M. López-Vivas & R. Riosmena-Rodríguez. 2016. New Record of the non-native seaweed Gracilaria parvispora in Baja California – A Note on Vergara-Rodarte et al. Cryptogamie, Algologie 37:257-263.
Kumar, M., P. Kumari, V. Gupta, C.R.K. Reddy & J. Bhavanath. 2010. Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. Journal of Experimental Marine Biology and Ecology 91: 27-34.
Labrada-Martagón, V., P.A. Rodríguez, L.C. Méndez-Rodríguez & T. Zenteno-Savín. 2011. Oxidative stress indicators and chemical contaminants in east Pacific green turtles (Chelonia mydas) inhabiting two foraging coastal lagoons in the Baja California peninsula. Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology 154: 65-75.
Lesser, M. 2006. Oxidative stress in marine environments: Biochemistry and Physiological Ecology. Annual Review of Physiology 68: 253-278.
Levine, R.L., J.A. Williams, E.P. Stadtman & E. Shacter. 1994. Carbonyl assays for determination of oxidatively modified proteins. In: L. Packer. Ed. Methods in Enzymology 233. Oxygen Radicals in Biological Systems Part C. Academic Press, New York, pp. 346-357.
Lohrmann, N., B. Logan & A. Johnson. 2004. Seasonal acclimatization of antioxidants and photosynthesis in Chondrus crispus and Mastocarpus stellatus, two co-occurring red algae with differing stress tolerances. Biology Bulletin 207: 225-232.
López-Cruz, R.I., T. Zenteno-Savín & F. Galván-Magaña. 2010. Superoxide production, oxidative damage and enzymatic antioxidant defenses in shark skeletal muscle. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 56: 50-56.
Maharana, D., P. Brata Das, X. N. Verlecar, N. M. Pise & M. U. Gauns. 2015. Oxidative stress tolerance in intertidal red seaweed Hypnea musciformis (Wulfen) in relation to environmental components. Environmental Science and Pollution Research 22: 18741-18749.
Nyberg, C. & I. Wallentinus. 2005. Can species traits be used to predict marine macroalgal introductions? Biological Invasions 7: 265-279.
Peltzer, D. & A. Polle. 2001. Diurnal fluctuations of antioxidative systems in leaves of field-grown beech trees (Fagus sylvatica): responses to light and temperature. Physiologia Plantarum 111: 158-164.
Persky, A.M., P.S. Green, L. Stubley, C.O. Howell, L. Zaulyanov, G.A. Brazeau & J.W. Simpkins. 2000. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proceedings of the Society for Experimental Biology and Medicine 223: 59-66.
Phooprong, S.I., H. Ogawa & K. Hayashizaki. 2007.Photosynthetic and respiratory responses of Gracilaria vermiculophylla (Ohmi) Papenfuss collected from Kumamoto, Shizuoka and Iwate, Japan. Journal of Applied Phycology 5: 293-300.
Pise, N.M., D.K. Gaikwad & T.G. Jagtap. 2013.Oxidative stress and antioxidant indices of the marine red alga Porphyra vietnamensis. Acta Botanica Croatica 72: 197-209.
Secretaría de Medio Ambiente y Recursos Naturales. 2017. Listado de Especies Exóticas Invasoras para México. https://www.gob.mx/cms/uploads/attachment/file/222541/Gracilaria_vermiculophylla.PDF
Singleton, V.L. & J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture 16: 144-158.
Suzuki, K. 2000. Measurement of Mn-SOD and Cu, Zn-SOD. In: N. Taniguchi & J. Gutteridge, Eds. Experimental Protocols for Reactive Oxygen and Nitrogen Species. Oxford University Press, Oxford pp. 91-95.
Tenorio-Rodriguez, P.A., J.I. Murillo-Álvarez, A.I. Campa-Cordova & C. Angulo. 2017. Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. Journal of Food Science and Technology 54: 422-429.
Thomsen, M.S. & K.J. McGlathery. 2007. Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. Biological Invasions 9: 499-513.
Tian, J. & J. Yu. 2009. Changes in ultrastructure and responses of antioxidant systems of algae (Dunaliella salina) during acclimation to enhanced ultraviolet-B radiation. Journal of Photochemistry and Photobiology B: Biology 97: 152-160.
Zar, J.H. 1999. Biostatistical Analysis. 4th ed. Prentice Hall, Englewood Cliffs.
Recibido: 20.09.18
Revisado: 03.12.18
Corregido: 19.12.18
Aceptado: 30.12.18
Revisores: 2 árbitros anónimos.