The Organellar Genomes of Melanthalia abscissa and Polyopes polyideoides (Rhodophyta, Florideophyceae).
Los genomas organelares de Melanthalia abscissa y Polyopes polyideoides (Rhodophyta, Florideophyceae).
Matthew N. Freiler1* & Juan M. Lopez-Bautista1,2
1 Department of Biological Sciences, The University of Alabama, 500 Hackberry Lane, Mary Harmon Bryant Hall #309, Tuscaloosa, AL 35487-0345.
2The University of Alabama Cuba Center for Collaboration and Scholarship, The University of Alabama, 1005 Math and Science Education Building, 411 Hackberry Lane, Tuscaloosa, AL 35487.
Email: mnfreiler@crimson.ua.edu
Freiler, M.N. & J.M. Lopez-Bautista. 2024. The Organellar Genomes of Melanthalia abscissa and Polyopes polyideoides (Rhodophyta, Florideophyceae). Cymbella 10(1-3): 05-18.
DOI: https://doi.org/10.22201/fc.24488100e.2024.10.1-3.1
Abstract
Florideophyceae is the most species rich red algal class, including large numbers of well-studied, economically important species and lesser-known clades. We present the complete plastid and mitochondrial genome assemblies of two Florideophycean species, Melanthalia abscissa (Gracilariaceae) and Polyopes polyideoides (Halymeniaceae). We identified more large-scale rearrangements within the plastid genomes of Gracilariales than within Halymeniales. Maximum likelihood phylogenies using rbcL data support the placement of both M. abscissa and P. polyideoides samples in monophyletic genera. However, not all genera within Halymeniales were recovered as monophyletic. Sequences that appear to be derived from red algal plasmids were identified within the plastid genome of M. abscissa. Determining the presence or absence of plasmid-derived sequences in the P. polyideoides plastome is more difficult due to a lack of publicly available data for Halymeniaceae. The addition of the sequences produced by this study will support further phylogenetic and systematic research on these Rhodophytan genera and orders.
Keywords: Florideophyceae, mitochondria, phylogenetics, plastid genome, rbcL.Resumen
Las Florideophyceae es la clase más numerosa en especies de las algas rojas, incluye un gran número de especies bien estudiadas económicamente importantes y algunos clados menos conocidos. En este trabajo presentamos los genomas completos plastidial y mitocondrial de dos especies de algas Florideophyceae, Melanthalia abscissa (Gracilariaceae) y Polyopes polyideoides (Halymeniaceae). Se identificaron más rearreglos a gran escala dentro de los genomas plastidiales de Gracilariales que dentro de las Halymeniales. Las filogenias por Máxima Probabilidad utilizando datos del rbcL apoyan la posición de ambas muestras, M. abscissa y P. polyideoides, dentro de géneros monofiléticos. Sin embargo, no todos los géneros dentro de Halymeniales fueron recuperados como monofiléticos. Secuencias que parecen ser derivadas de plásmidos de algas rojas se identificaron dentro del genoma plastidial de M. abscissa. La determinación de la presencia o ausencia de secuencias derivadas de plásmidos en el plastoma de P. polyideodies es más difícil debido a la ausencia de datos públicamente disponibles para las Halymeniaceae. La adición de las secuencias producidas en este estudio apoyará futuras investigaciones filogenéticas y sistemáticas en estos géneros y ordenes de Rhodophyta.
Palabras clave: filogenética, Florideophyceae, genoma plastidial, mitocondria, rbcL.Introduction
Rhodophyta is a monophyletic division of red algae that consists of seven classes. Florideophyceae, the most species rich class in the division, contains ~7,100 of the ~7,500 Rhodophytan species (Guiry 2017). The class has a considerable impact on multiple sectors of the human economy. The commercially used agarophytes, carrageenophytes, and several economically important edible seaweeds belong to Florideophyceae. Members of the Florideophyceae also hold major importance to ocean ecosystems. Corallinales, a resilient and widespread order of crustose Florideophyceans, are critical builders of coral reef frameworks in the global tropics (Bjork et al. 1995). Despite this economic and ecological importance, several branches of subordinate Florideophyceae taxonomy rely entirely on analyzing morphological features, especially the reproductive structures (De Clerck et al. 2012). The uncertain inter-ordinal relationships may be resolved by further implementing phylogenomic approaches.
Organellar genomes contain useful sequence data for developing reliable phylogenies. Compared to nuclear genomes, their high copy and short length make extraction, assembly, and analysis straight-forward. Plastids and mitochondria are typically inherited uniparentally, gene-dense, and highly conserved. Due to their conserved nature, organellar DNA can be particularly valuable in resolving deep-branching phylogenies (Palmer et al. 1988).
Less than three percent of Florideophyceans have plastid genomes available on RefSeq, NCBI’s non-redundant database, with only 149 published (O’Leary et al. 2016). Using a matrix of mostly plastid coding and ribosomal loci, as well as a selection of nuclear coding and ribosomal loci, Verbruggen et al. (2010) identified five notable regions of low support in the Rhodophytan phylogeny. Substantial increases have been made in the availability of the loci used by Verbruggen et al. since the study’s release in 2010. However, much of the red algal phylogeny remains unresolved (Díaz-Tapia et al. 2018). The addition of sequence data will provide better resolution of these regions of the phylogeny.
In this study, we focus on the plastid and mitochondrial genomes of two species, Melanthalia abscissa (Turner) Hooker f. & Harvey and Polyopes polyideoides Okamura, from two Florideophycean orders, Gracilariales and Halymeniales, respectively.
Melanthalia Montagne is a genus of only four species within the family Gracilariaceae, which contains agarophytan genera such as Gracilaria Greville and Gracilariopsis E.Y. Dawson. Despite the variety of known applications for Gracilaria, Melanthalia is not used as extensively.
Currently, the genus has minimal usage in aquaculture. It is not used commercially for agar production (Furneaux et al. 1990) or for human consumption. Laboratory experiments with extracts from M. abscissa have increased larval settlement of Perna canaliculus, a commonly cultivated mussel in New Zealand aquaculture (Alfaro et al. 2006). Melanthalia has a small geographic range and limited distribution in the subtidal waters of New Zealand and Australia (Nelson et al. 2013). A plastid genome from one member of the genus, Melanthalia obtusata (Labillardière) J. Agardh is available on GenBank under the synonymous name, Melanthalia intermedia. The systematics of this genus and corresponding species within it have changed multiple times in recent years and remains elusive (Iha 2018, Lyra 2021, Nelson et al. 2013).
Polyopes J. Agardh is a small genus of eleven species within the family Halymeniaceae (Guiry 2017). It is native to East Asia and particularly prevalent in the coastal waters of Japan. However, it has recently appeared on European coasts, likely through accidental introduction from human activity (Mineur et al. 2010). Phylogenomic analyses of the genus have been limited, though it has gained some attention for the various pharmaceutical applications of Polyopes affinis (Harvey) Kawaguchi & Wang and Polyopes lancifolius (Harvey) Kawaguchi & Wang. Ethanol extracts from P. affinis have shown potential in treating airway inflammation in mice and human asthma models (Lee et al. 2011; Ha et al. 2022), as well as acting as photoprotection against ultraviolet-B light on human cells (Hyun et al. 2014). P. lancifolius has been used to treat high blood sugar in diabetic mice (Kim et al. 2010). At the date of writing, only eight unique plastid genomes from Halymeniales are available on GenBank. From these, three correspond to the family Halymeniaceae.
The goal of this study is to add to the limited knowledge base of the Florideophycean genera Melanthalia and Polyopes, by providing complete organellar genome sequences, and examining the characteristics of these genomes. Publication of organellar genomes from clades underrepresented in databases will benefit future species identifications, systematic and ecological investigations, and conservation of this critical class. The Florideophyceae are crucial to the global ecosystem, acting as keystone reef builders and invasive species.
Methodology
M. abscissa specimens were collected by D. Wilson Freshwater in 1994 from a subtidal habitat near the Mataikona river in Wairarapa, New Zealand. The voucher specimen is stored at the Te Papa Tongarewa Museum of New Zealand Herbarium with the voucher number WELT A024150. DNA is in the algal DNA collection at The University of Alabama under the number UA 816. P. polyideoides specimens were collected by Suzanne Fredericq in Keelung City, Taiwan, in 1993. DNA is stored at the University of Alabama under the number UA 733. Sequencing was performed at Cold Spring Harbor Laboratory (Cold Spring Harbor, New York, USA) on the Illumina MiSeq Platform. For the M. abscissa sample, 2,159,473 paired-end reads (101 bp) were produced. For the P. polyideoides sample, 4,732,738 paired-end reads were produced.
Plastid genomes were assembled and subsequently analyzed on The University of Alabama High-Performance Computing (HPC) cluster using the following steps. Initial read quality was examined using FastQC v0.11.5 (Andrews 2010) before cleaning and trimming with Trimmomatic v0.4 (Bolger et al. 2014) with minimum leading and trailing quality scores of 20 and a minimum read length of 65. Following trimming, normalization to 100x read coverage to reduce any possible overrepresented sequences was done using BBnorm (Bushnell 2014). De novo assembly was performed by SPAdes v3.14 (Prjibelski et al. 2020) using the ‘plasmid’ and ‘careful’ options. A database of published Rhodophyta organellar genes and complete organellar genomes was created with BLASTn 2.9.0 (Altschul et al. 1990) to select the scaffolds containing the organellar genomes. Seed based microassembly tool, afin (Wilson 2016) was needed to extend and finish the plastome of P. polyideoides. Coverage analyses were performed by Fast-Plast v1.2.9 (McKain & Wilson 2017) to evaluate the assembly. Protein coding sequences were identified by Plastid Genome Annotator (Qu et al. 2019), and ribosomal genes and tRNAs within the plastid genome were detected by Chlorobox GeSeq (Tillich et al. 2019) and tRNAscan-SE 2.0 (Lowe & Chan 2016). Gene lengths and reading frames were manually checked by aligning each protein product to the closest homolog. Any necessary edits to the automated annotations were made in Geneious V.2023.0.4 (Geneious 2023) and UGENE V.47 (Okonechnikov et al. 2012).
Open reading frames (ORFs) of 25 amino acids or longer in intergenic space with BLAST matches to other members of Rhodophyta were retained in the annotation. BLASTp searches of the non-redundant database of Rhodophyta data were used. An E-value of 1e-5 and percent identity cut off of 60% were used to determine potential homology with ORFs in other Rhodophyta plastomes. ORFs were named based on the length of their potential amino acid product. Microsatellites, or simple sequence repeats (SSRs), were identified by the MIcroSAtellite (MISA) identification tool v.2.1 (Beier et al. 2017). The minimum number of repeats to detect microsatellites was set to ten for mononucleotide repeats, six for dinucleotide repeats, and five for trinucleotide repeats or anything larger. These settings are the default option on the MISA web server. These parameters have also been used in other studies analyzing microsatellites in algal plastid genomes (Kuntal et al. 2012). Visualization of complete organellar genomes was done by Chlorobox OGDRAW (Greiner et al. 2019).
Complete coding sequences of rbcL, extracted from plastid genome assemblies were aligned by MAFFT v7.313 (Katoh & Stanley 2013) with plastid genome data available on GenBank. To further assess the systematic position of the algal samples, two maximum likelihood phylogenies were constructed using this data, in IQ-Tree v2.2.0 (Minh et al. 2020). Substitution models were chosen using the “auto” option in IQ-Tree. The TIM2+F+I+G4 model was chosen for the Gracilariales phylogeny, while the TIM2+F+G4 model was chosen for the Halymeniales phylogeny. The Halymeniales phylogeny was constructed with all rbcL sequences from the order with a length over 1400 base pairs available on GenBank. Due to the small number of complete plastid genomes published on GenBank, the use of data solely from complete assemblies would not construct a sufficient phylogeny of the order.
Tree visualization was done using FigTree v1.4.4 (Rambaut 2010), Adobe Illustrator v28.2 and Acrobat v23 (Adobe Inc. 2024, Adobe Inc. 2024). Plastome rearrangements between both novel sequences and their respective orders were examined through Mauve (Darling et al. 2004) genome alignments. Representatives of each genus present in the Halymeniales and Gracilariales phylogenies were selected for alignment, along with the sequences from this study. Alignments were performed by progressive Mauve using the default parameters and HOXD scoring. Start positions for each alignment were standardized to the most common position in the taxon sampled. The Gracilariales alignment begins at rns while the Halymeniales alignment begins at rbcL. Possible plasmid-derived sequences (PDS) were identified by similarity to a custom BLASTn database of Rhodophyta plasmid sequences.
Results
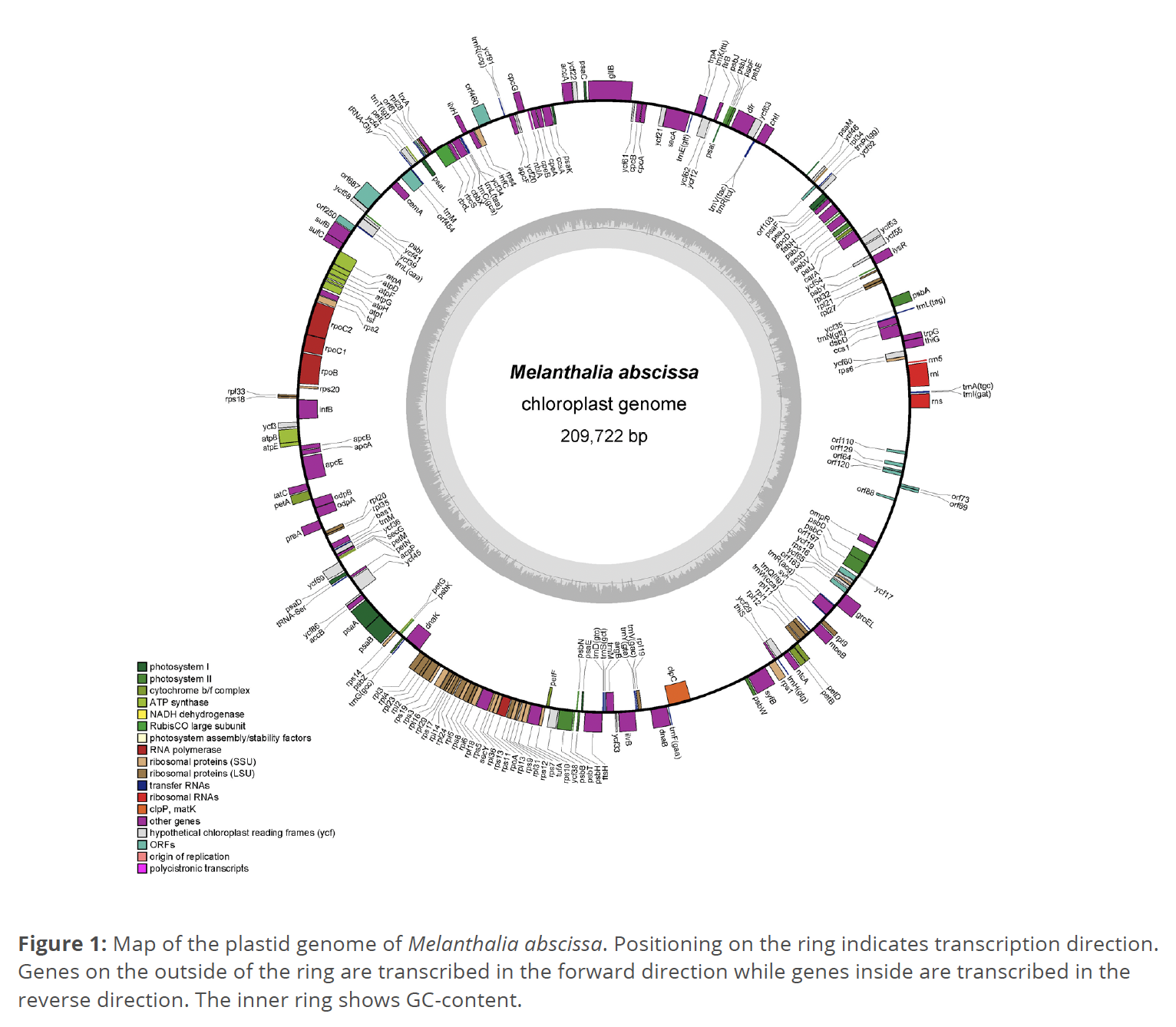
The assembled M. abscissa plastid genome has a length of 209,722 base pairs, and GC-content of 32.2% (Fig. 1). The average depth of coverage for the assembly is 55. A total of 190 protein-coding genes were identified along with 29 tRNA sequences and three rRNA genes, and 15 ORFs. Identified genes with overlap include lysR with ycf54, sufB with sufC, atpD with atpF, rpl33 with rps18, ycf59 with leuD, rpl4 with rpl23, rpl14 with rpl24, and psbC with psbD. Including ORFs identified, protein-coding regions account for 68.4% of the plastome.
Two simple sequence repeats (SSR) were found in the plastome of M. abscissa. A trinucleotide repeat of AGC was found at positions 11,417 to 11,431 within the psbA gene, and a dinucleotide repeat of AT was found at positions 174,075 to 174,088 within the syfB gene. No inverted repeat was found. Mauve alignments of the entire plastomes show locally collinear block (LCB) rearrangement is observed in the alignment between Melanthalia abscissa and Melanthalia obtusata. The plastid genome of M. abscissa is published on GenBank under the accession number PP328475.
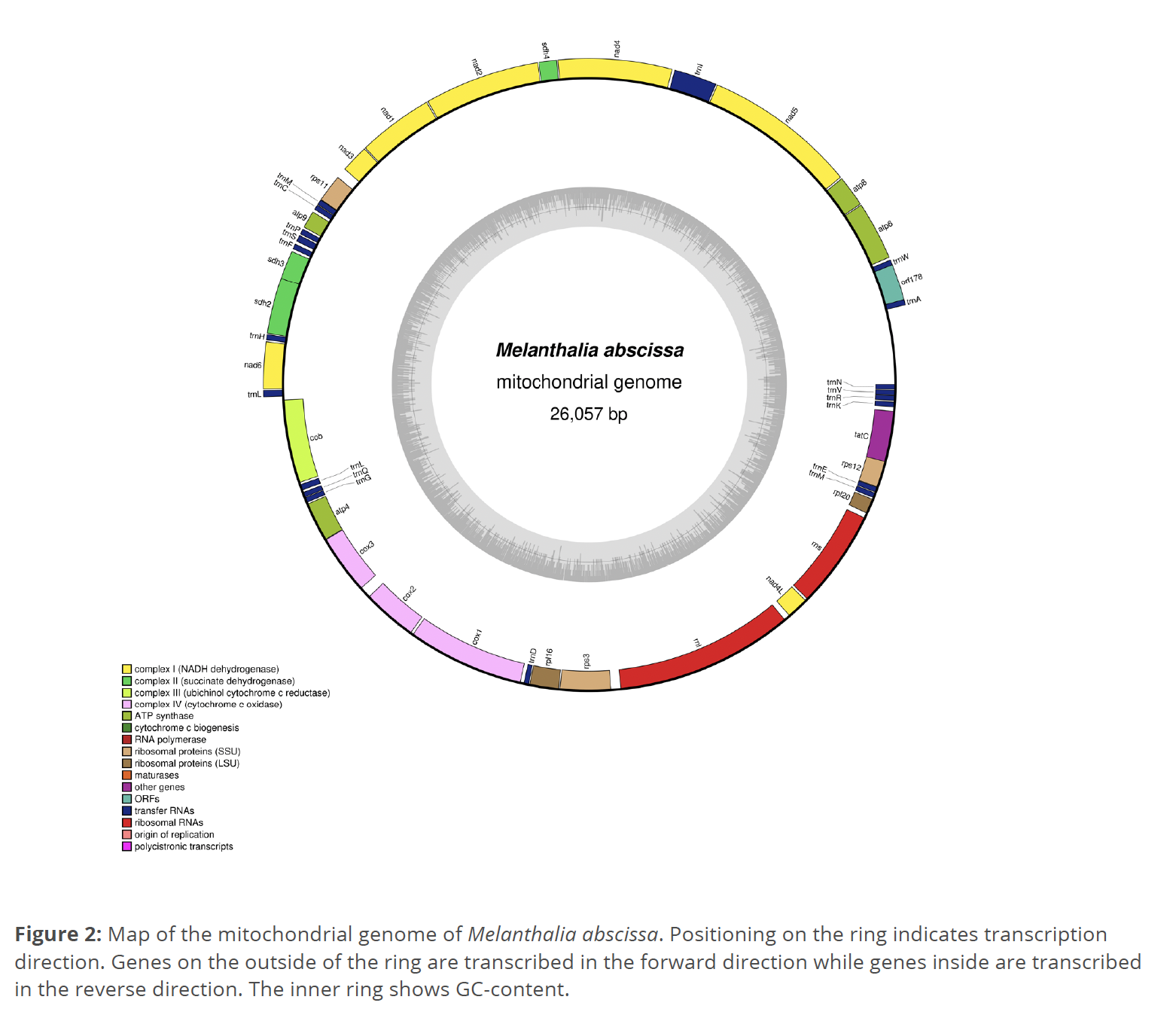
A mitochondrial genome 26,057 base pairs long with a GC content of 30.1% was assembled (Fig. 2). The average depth of coverage for the assembly is 75. The mitochondrial genome contains 24 protein-coding genes, one 537 base- pair ORF, and 20 tRNAs. No SSRs were found by MISA in the M. abscissa mitochondrial genome. Starting from the trnA gene, all genes occur on the forward strand until the trnL gene around the midpoint of the genome. All genes between cob and trnN occur on the reverse strand. The mitochondrial genome of M. abscissa is published on GenBank under the accession number PP335805.
The P. polyideoides plastome comprises 201,550 base pairs, 192 protein coding genes, and 29, tRNA sequences, three rRNA sequences, and 17 ORFs (Fig. 3). The average depth of coverage for the assembly is 230. Like M. abscissa, a few identified genes have overlapping regions, including rpl24 with rpl14, rpl23 with rpl4, trpG with ccs1, and psbD with psbC. Protein coding regions account for 74.2% of the plastome.
Five SSRs were located in the plastid genome of Polyopes polyideoides. A monomeric repeat of A was found within the rps1 gene at positions 179,843 to 179,852. Monomeric repeats of T were found at positions 146,373 to 146,382 within the rpl21 gene as well as 195,608 to 195,617 at the end of ycf33. A dinucleotide repeat of AT is present at positions 185,213 to 185,224, and a dinucleotide repeat of TA from 108,513 to 108,524.
No large inverted repeats were found. However, ORFs 122 and 121 at the beginning of the plastome are identical for the first 313 base pairs. The ORFs are 377 and 366 base pairs in length respectively. Sparse rearrangements between P. polyideoides and the rest of Halymeniales are seen in the Mauve alignment. The plastid genome of P. polyideoides is published on GenBank under the accession number PP338773.
The P. polyideoides mitochondrial genome is 26,499 base pairs long and has a GC-content of 30.7% (Fig. 4). The average depth of coverage for the assembly is 174. Three SSRs were identified in the mitochondrial genome of P. polyideoides. None of them were in coding regions. A trinucleotide of AGT is present from positions 26,071 to 26,085. Dinucleotide repeats of TA and AT can be found at positions 26,162 to 26,175 and 26,221 and 26,238, respectively.
Like the M. abscissa mitochondrial genome, 24 protein-coding genes and one ORF are present. However, four additional tRNAs were found, for a total of 24. The gene order and direction are the same as seen in the mitochondrial genome of M. abscissa. The mitochondrial genome of P. polyideoides is published on GenBank under the accession number PP338774.
The phylogenetic analysis placed the novel M. abscissa plastome within Melanthalia alongside the sister species M. obtusata with a bootstrap value of 100 (Fig. 5). All genera sampled from Gracilariales formed monophyletic clades. However, there are varying degrees of support for these nodes.
Due to the sparse number of complete plastid genome assemblies from Halymeniales on RefSeq, full-length rbcL sequences could not be used to construct a useful phylogeny in the same manner as Gracilariales. A larger phylogeny with 31 taxa and an outgroup, was able to be constructed, but with a widely varying support (Fig. 6). Halymenia floridana was placed in a well-supported clade with members of the genus Cryptonemia rather than with the other member of Halymenia in the phylogeny, H. maculata.
Large scale rearrangements were examined using Mauve alignments of complete plastid genomes from representatives of each genus from Gracilariales and Halymeniales with plastomes available on RefSeq. Halymeniales plastid genomes are strongly conserved in order. Most of each plastome is contained within one locally collinear block (LCB), with no further rearrangements (Fig. 7). Smaller LCBs are present at the ends of the genomes. This contrasts the rearrangement observed in the Gracilariales alignment, particularly in the genus Melanthalia (Fig. 8). White space within the LCBs signifies regions unique to that genome compared to the others in the alignment.
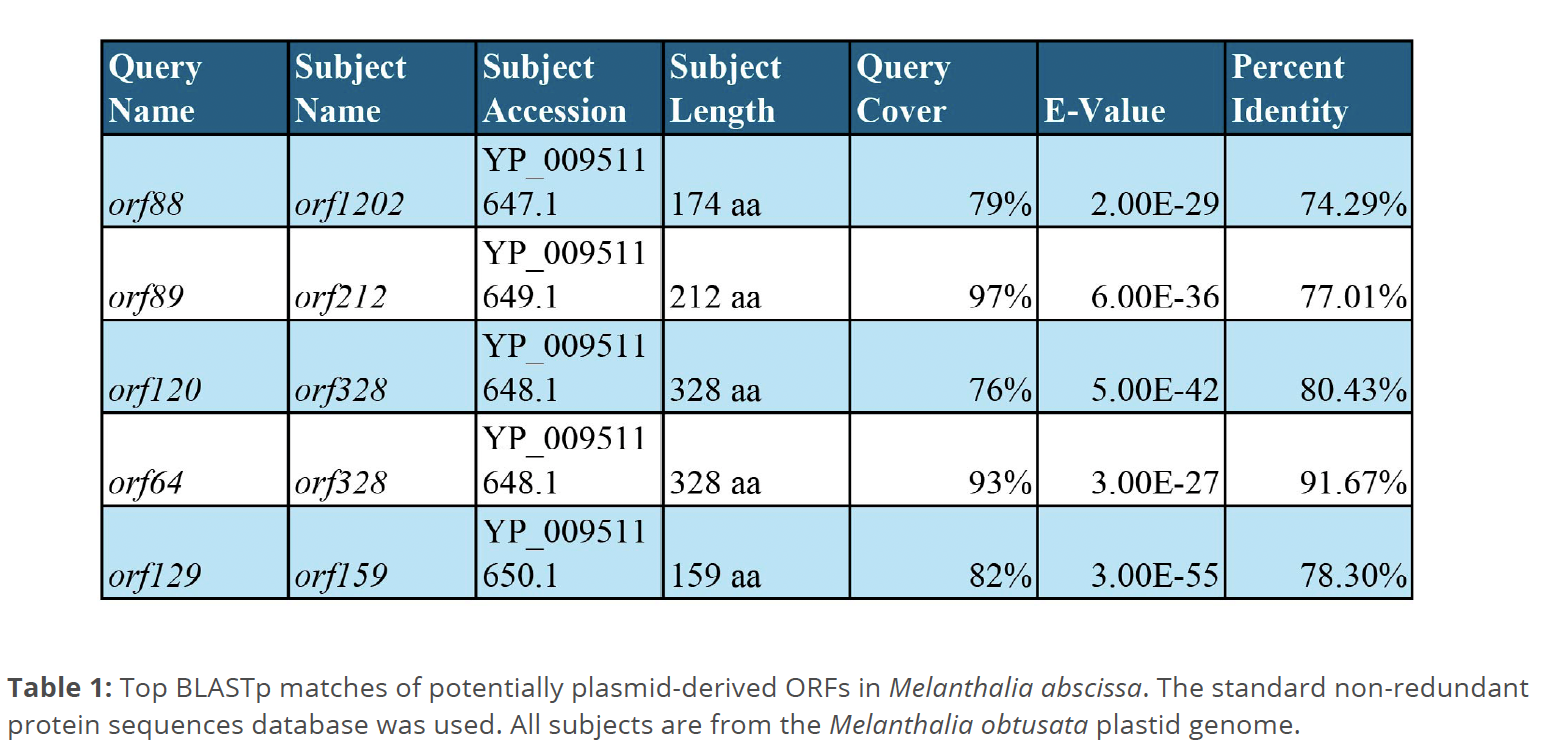
BLASTn searches show the plasmid GL3.5 from Gracilaria lemaneiformis (Goff & Coleman 1990), matching short sequences of the M. abscissa plastome in three separate locations, within these unique regions (positions 30,463 to 31,076, 199,377 to 199,608, and 202,884 to 203,362). Two of these locations contain ORFs (orf88 and orf129). Similarity to the plasmid Gve4548 from Gracilaria vermiculophylla was also found in one sequence (positions 205,209 to 205,381).
The ORFs orf88, orf89, orf73, orf120, orf64, and orf129 return a similarity to ‘plasmid- derived’ ORFs in Melanthalia obtusata in BLASTp searches of the RefSeq database, but do not match any plasmid genes directly (Table 1). No plasmid matches were found in P. polyideoides.
Discussion
The 209,722 base pair length of the M. abscissa plastid genome makes it the second longest within the Florideophyceae sequenced to date, just behind M. obtusata. Their plastomes are also the most GC-rich plastomes of the Gracilariales. M. abscissa has the highest GC-content of the order at 32.2%. With a length of 201,550, P. polyideoides is the third longest within the class, and 5,377 base-pairs longer than the other published member of its genus P. affinis. Neither plastome was found to possess inverted repeats. This is consistent with other published members of Melanthalia and Polyopes.
Microsatellites, or simple sequence repeats (SSRs) in the chloroplasts of red algae have been used to distinguish between individuals from different geographical locations (Song et al. 2014). SSRs are also useful for diversity, gene flow, and evolutionary studies. (Vieira et al. 2016). Both of the SSRs identified in the M. abscissa plastid genome are located within the coding regions of genes. The trinucleotide repeat found in the psbA gene of M. abscissa results in a repeat of alanine in the translation. This repeat is also found in the same gene of the M. obtusata plastid genome. The monomeric repeat within the rpl21 gene of P. polyideoides can also be found within the same gene in the plastid genome of P. affinis. The retention of these repeats across different species may be functionally significant, as mutation rates of tandem repeats is typically much higher than the rest of the genome (Gemayel et al. 2012).
Phylogenetic analysis confirms the positions of our samples of M. abscissa and P. polyideoides within the genera Melanthalia and Polyopes, respectively. All genera represented in the Gracilariales phylogeny are monophyletic (Fig. 5). However, not all families of Halymeniales represented were recovered as monophyletic (Fig. 6). Halymeniaceae excludes Yonagunia S. Kawaguchi & M. Masuda and Grateloupia C. Agardh, which belong to Grateloupiaceae though they share a common ancestor with Polyopes and Glaphyrosiphon Hommersand & Leister, members of Halymeniaceae. Both Yonagunia and Grateloupia were previously considered members of Halymeniaceae until the resurrection of the family Grateloupiaceae was supported by an extensive, multigene phylogeny from 47 taxa (Kim et al. 2021). The resurrection of Grateloupiaceae is controversial and disputed on a morphological basis (Nguyen et al. 2023). Further studies are needed to clarify their systematic position.
Halymenia C. Agardh was the only genus found as non-monophyletic in our phylogeny. Halymenia floridana J. Agardh was placed with strong bootstrap support within Cryptonemia J. Agardh rather than with the other member of Halymenia, H. maculata J. Agardh. This is not surprising since extensive rbcL phylogenies have shown both Cryptonemia and Halymenia to be polyphyletic (Rodríguez-Prieto et al. 2018). Specimens of H. floridana from Brazil do not exhibit the summer seasonality or subtidal habitat of other species of Halymenia, but rather the all-season presence and intertidal habitat of Cryptonemia (Azvedo et al. 2016). Furthermore, other rbcL phylogenies have placed it within Cryptonemia (Azvedo et al. 2018, Rodríguez-Prieto et al. 2018). Despite the support for the transfer of H. floridana to Cryptonemia, it remains a member of Halymenia due to a lack of analysis of specimens from Florida, the type locality (Schneider et al. 2018). The specimen included in our phylogeny presented here is from Brazil, which may not be the same species as the true H. floridana.
Prior to this study, only one complete plastid genome from Polyopes was available on GenBank. The represented members of the genus in the phylogeny presented here form a monophyletic genus. The rbcL sequence from our sample of P. polyideoides was placed as a sister clade to P. affinis, with P. lancifolius diverging earlier. The intra-ordinal relationships of Halymeniales remain uncertain, with several genera such as Halymenia and Grateloupia not appearing as monophyletic in rbcL or multigene phylogenies (Kim et al. 2021).
No LCB rearrangement occurred between the plastomes of the two members of Polyopes (Fig. 8). This was not the case in Melanthalia; where two LCBs are relocated, one of which is reversed. M. abscissa and M. obtusata also exhibit more regions unique to their plastome compared to the other members of Gracilariles (Fig. 7). Unique regions appear more commonly in the plastomes of Halymeniales. In Halymeniales, many of these regions lacking similarity appear in the same approximate position in the plastome. These regions may be the result of plasmid insertions (Ng et al. 2017). Plasmids that originate and self-replicate within red algae have been observed in other Florideophycean plastid genomes (Iha et al. 2018, Ng et al. 2017). An early characterization of plasmids in the genome of Gracilaria did not find any exchange between plasmids and the nuclear or organellar genomes (Goff & Coleman 1990). However, more recent studies have found evidence for the movement of DNA from red algal plasmids to mitochondrial and plastid genomes (Lee et al. 2016).
Plasmid-derived sequences (PDS) appear to be present in the plastid genome of M. abscissa. Short, nucleotide sequences that matched circular plasmids from other members of Gracilariales were found in the regions of the genome that are unique according to the Mauve alignment. Some of these locations contain ORFs, and multiple ORFs were found to have similarities to ORFs designated as ‘plasmid-derived’ in the plastid genome of Melanthalia obtusata. These sites are potentially the result of horizontal gene transfer. However, no direct matches to plasmid ORFs or plasmid protein coding genes were found in the plastome. No sequences were found to match plasmids in the plastome of P. polyideoides. This may be due to genuine absence of PDS, or lack of reference data. No plasmids from Halymeniales have been published on GenBank to date, compared to the eight published from Gracilariales.
Gene order and direction are identical in the Melanthalia abscissa and Polyopes polyideoides mitochondrial genomes. The split directionality of these mitochondrial genomes is commonly seen in other mitogenomes of Florideophyceae (Iha et al. 2018). Possible reasons for this occurrence do not appear well documented and warrant further investigation.
Conclusions
The subfamily Melanthalioideae that contains Melanthalia was determined to be the sister group to the rest of Gracilariaceae based on rbcL phylogenies and morphological data (Gurgel et al. 2018). As found in this study, the genus Melanthalia has characteristically large and GC-rich plastid genomes compared to the rest of Gracilariales. Sequence data for the other genus belonging to Melanthalioideae, Curdiea is very limited. No complete plastid genomes have been published to date. Mauve alignments also show more rearrangement between Melanthalia plastid genomes and the other representatives of the order. The early divergence from the rest of Gracilariales, and their limited geographic range may contribute to these unique characteristics.
Limited plastid sequence data for Halymeniales makes drawing conclusions for Polyopes more problematic. However, rearrangements between members of the order seem to occur less frequently than in Gracilariales. The contribution of more organellar genomes and plasmid sequences is needed for a more comprehensive analysis of this order. The data presented here adds to the limited knowledge base of both Melanthalia and Polyopes, as well as their respective ordinal status.
Data Availability
Organellar genomes are published on GenBank under the accession numbers, PP328475, PP335805, PP338773, and PP338774. The voucher specimen for Melanthalia abscissa is located at the Te Papa Tongarewa Museum of New Zealand Herbarium in Wellington, New Zealand with the voucher number WELT A024150. DNA from this specimen stored at The University of Alabama PhycoLab under the number UA 816. DNA for P. polyideoides is stored under the number UA 733.
Acknowledgments
We want to express our gratitude to Drs. Michael R. McKain (UA), James T. Melton (Spelman College), and Daryl W. Lam (UA) for their suggestions on improving this manuscript.
REFERENCIAS
Adobe Inc. 2024. Adobe Acrobat Version 23.008.20533.
Adobe Inc. 2024. Adobe Illustrator Version 28.2.
Alfaro, A.C., B.R. Copp, D.R. Appleton, S. Kelly, & A.G. Jeffs. 2006. Chemical cues promote settlement in larvae of the green-lipped mussel, Perna canaliculus. Aquaculture International 14: 405-412.
Altschul, S.F., W. Gish, W. Miller, E.W. Myers, & D.J. Lipman. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403-410.
Andrews, S. 2010. FastQC: A Quality Control Tool for High Throughput Sequence Data.
[Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Azevedo, C.A.A.D., V. Cassano, & M.C. Oliveira. 2016. Phylogenetic relationships among Halymenia (Halymeniaceae, Rhodophyta) species on the Brazilian coast with description of Halymenia cearensis sp. nov. Phytotaxa 280: 241-258.
Beier, S., T. Thiel, T. Münch, U. Scholz, & M. Mascher. 2017. MISA-web: a web server for microsatellite prediction, Bioinformatics 33: 2583–2585, https://doi.org/10.1093/bioinformatics/btx198.
Bjork, M., S.M. Mohammed, M. Bjorklund, & A. Semesi. 1995. Coralline algae, important coral-reef builders threatened by pollution. Ambio 24: 502-505.
Bolger, A.M., M. Lohse, & B. Usadel. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114-2120.
Bushnell, B. 2014. BBMap: a fast, accurate, splice-aware aligner. 9th Annual Genomics of Energy & Environment Meeting, Walnut Creek, CA, United States.
Darling, A.C., B. Mau, F.R. Blattner, & N.T. Perna. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Research 14: 1394-1403.
De Clerck, O., K.A. Bogaert, & F. Leliaert. 2012. Diversity and evolution of algae: primary endosymbiosis. Advances in Botanical Research 64: 55-86.
Díaz-Tapia, P., M.M. Pasella, H. Verbruggen, & C.A. Maggs. 2019. Morphological evolution and classification of the red algal order Ceramiales inferred using plastid phylogenomics. Molecular Phylogenetics and Evolution 137: 76-85.
Furneaux, R.H., I.J. Miller, & T.T. Stevenson. 1990. Agaroids from New Zealand members of the Gracilariaceae (Gracilariales, Rhodophyta)—a novel dimethylated agar. In Thirteenth International Seaweed Symposium: Proceedings of the Thirteenth International Seaweed Symposium held in Vancouver, Canada, August 13–18, 1989 (pp. 645-654). Springer Netherlands.
Gemayel, R., J. Cho, S. Boeynaems, & K.J. Verstrepen. 2012. Beyond junk-variable tandem repeats as facilitators of rapid evolution of regulatory and coding sequences. Genes 33: 461-480.
Geneious Prime 2023.0.1 (https://www.geneious.com).
Goff, L.J. & A.W. Coleman. 1990. Red algal plasmids. Current Genetics 18: 557-565.
Greiner, S., P. Lehwark, & R. Bock. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Research 47: W59-W64.
Gurgel, C.F.D., J.N. Norris, W.E. Schmidt, H.N. Le, & S. Fredericq. 2018. Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 374: 1-23.
Guiry, M.D. & G.M. Guiry. 2017. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org
(accessed on March 6, 2017).
Ha, Y., W.H. Lee, J.K. Kim, H.K. Jeon, J. Lee, & Y.J. Kim. 2022. Polyopes affinis Suppressed IFN-γ-and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules 27: 1836.
Hyun, Y.J., M.J. Piao, K.C. Kim, J. Zheng, C.W. Yao, J.W. Cha, & J.W. Hyun. 2014. Photoprotective effect of a Polyopes affinis (Harvey) Kawaguchi and Wang (Halymeniaceae)-derived ethanol extract on human keratinocytes. Tropical Journal of Pharmaceutical Research 13: 863-871.
Iha, C., C.J. Grassa, G.D.M. Lyra, C.C. Davis, H. Verbruggen, & M.C. Oliveira. 2018. Organellar genomics: a useful tool to study evolutionary relationships and molecular evolution in Gracilariaceae (Rhodophyta). Journal of Phycology 54: 775-787.
Katoh, K. & D.M. Standley. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30:772-80. doi: 10.1093/molbev/mst010. Epub PMID: 23329690; PMCID: PMC3603318.
Kim, K.Y., T.H. Nguyen, H. Kurihara, & S.M. Kim. 2010. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. Journal of Food Science 75: H145-H150.
Kim, S.Y., H.W. Lee, E.C. Yang, S.M. Boo, J. Lopez-Bautista, S. Fredericq, & M.S. Kim. 2021. Resurrection of the family Grateloupiaceae Emend.(Halymeniales, Rhodophyta) based on a multigene phylogeny and comparative reproductive morphology. Frontiers in Ecology and Evolution 9: 775627.
Kuntal, H., V. Sharma, & H. Daniell. 2012. Microsatellite analysis in organelle genomes of Chlorophyta. Bioinformation 8: 255.
Lee, D.S., W.S. Park, S.J. Heo, S.H. Cha, D. Kim, Y.J. Jeon, & W.K. Jung. 2011. Polyopes affinis alleviates airway inflammation in a murine model of allergic asthma. Journal of Biosciences 36: 869-877.
Lee, J., K.M. Kim, E.C. Yang, K.A. Miller, S.M. Boo, D. Bhattacharya, & H.S. Yoon. 2016. Reconstructing the complex evolutionary history of mobile plasmids in red algal genomes. Scientific Reports 6: 23744.
Lowe, T.M. & P.P. Chan. 2016. tRNAscan-SE On-line: Search and Contextual Analysis of
Transfer RNA Genes. Nucleic Acids Research 44: W54-57.
Lyra, G.D.M., C. Iha, C.J. Grassa, L. Cai, H. Zhang, C. Lane, & C.C. Davis. 2021. Phylogenomics, divergence time estimation and trait evolution provide a new look into the Gracilariales (Rhodophyta). Molecular Phylogenetics and Evolution 165: 107294.
McKain, M. & M. Wilson. 2017. Fast-Plast: Rapid de novo assembly and finishing for whole chloroplast genomes. Available form: https://github.com/mrmckain/Fast-Plast. Phylogenetics and Evolution 43: 1118-1130.
Mineur, F., O. De Clerck, A. Le Roux, C.A. Maggs, & M. Verlaque. 2010. Polyopes lancifolius (Halymeniales, Rhodophyta), a new component of the Japanese marine flora introduced to Europe. Phycologia 49: 86-96.
Minh, B.Q., H.A. Schmidt, O. Chernomor, D. Schrempf, M.D. Woodhams, A. von Haeseler, & R. Lanfear. 2020. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology Evolution 37: 1530-1534. https://doi.org/10.1093/molbev/msaa01.
Nelson, W.A., C.E. Payri, J.E. Sutherland, & J. Dalen. 2013. The genus Melanthalia (Gracilariales, Rhodophyta): new insights from New Caledonia and New Zealand. Phycologia 52: 426-436.
Ng, P.K., S.M. Lin, P.E. Lim, L.C. Liu, C.M. Chen, & T.W. Pai. 2017. Complete chloroplast genome of Gracilaria firma (Gracilariaceae, Rhodophyta), with discussion on the use of chloroplast phylogenomics in the subclass Rhodymeniophycidae. BMC genomics 18: 1-16.
Nguyen, X.V., L.M. Liao, & S.M. Lin. 2023. Systematic revision of the Grateloupia complex (Rhodophyta) from Vietnam using rbcL and LSU sequences, with the description of Phyllymenia nhatrangensis sp. nov. Phycologia 62: 436-444.
O’Leary, N.A., M.W. Wright, J.R. Brister, S. Ciufo, D. Haddad, R. McVeigh, B. Rajput, B. Robbertse, B. Smith-White, D. Ako-Adjei, A. Astashyn, A. Badretdin, Y. Bao, O. Blinkova, V. Brover, V. Chetvernin, J. Choi, E. Cox, O. Ermolaeva, C.M. Farrell, T. Goldfarb, T. Gupta, D. Haft, E. Hatcher, W. Hlavina, V.S. Joardar, V.K. Kodali, W. Li, D. Maglott, P. Masterson, K.M. McGarvey, M.R. Murphy, K. O’Neill, S. Pujar, S.H. Rangwala, D. Rausch, L.D. Riddick, C. Schoch, A. Shkeda, S.S. Storz, H. Sun, F. Thibaud-Nissen, I. Tolstoy, R.E. Tully, A.R. Vatsan, C. Wallin, D. Webb, W. Wu, M.J. Landrum, A. Kimchi, T. Tatusova, M. DiCuccio, P. Kitts, T. D. Murphy, & K.D. Pruitt. 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Research 4: D733-45.
Okonechnikov, K., O. Golosova, M. Fursov, & Ugene Team. 2012. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28: 1166-1167.
Palmer, J.D., R.K. Jansen, H.J. Michaels, M.W. Chase, & J.R. Manhart. 1988. Chloroplast DNA variation and plant phylogeny. Annals of the Missouri Botanical Garden 75: 1180-1206.
Prjibelski, A., D. Antipov, D. Meleshko, A. Lapidus, & A. Korobeynikov. 2020. Using SPAdes de novo assembler. Current Protocols in Bioinformatics 70: e102.
Qu, X-J, M.J. Moore, D-Z Li, & T-S Yi. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 15:50.
Rambaut, A. 2010. FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/.
Rodríguez-Prieto, C., O. De Clerck, J.M. Huisman, & S.M. Lin. 2018. Systematics of the red algal genus Halymenia (Halymeniaceae, Rhodophyta): characterization of the generitype H. floresii and description of Neofolia rosea gen. et sp. nov. European Journal of Phycology 53: 520-536.
Schneider, C.W., C.E. Lane, & G.W. Saunders. 2018. A revision of the genus Cryptonemia (Halymeniaceae, Rhodophyta) in Bermuda, western Atlantic Ocean, including five new species and C. bermudensis (Collins & M. Howe) comb. nov. European Journal of Phycology 53: 350-368.
Shimodaira, H. & M. Hasegawa. 2001. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17: 1246-1247.
Song, S.L., P.E. Lim, S.M. Phang, W.W. Lee, D.D. Hong, & A. Prathep. 2014. Development of chloroplast simple sequence repeats (cpSSRs) for the intraspecific study of Gracilaria tenuistipitata (Gracilariales, Rhodophyta) from different populations. BMC Research Notes 7: 1-9.
Tillich, M., P. Lehwark, T. Pellizzer, E.S. Ulbricht-Jones, A. Fischer, R. Bock, & S. Greiner. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Research 45: W6-W11.
Verbruggen, H., C.A. Maggs, G.W. Saunders, L.L. Gall, H.S. Yoon, & O. De Clerck. 2010. Data mining approach identifies research priorities and data requirements for resolving the red algal tree of life. BMC Evolutionary Biology 10: 16. https://doi.org/10.1186/1471-2148-10-16.
Vieira, M.L.C., L. Santini, A.L. Diniz, & C.D.F. Munhoz. 2016. Microsatellite markers: what they mean and why they are so useful. Genetics and Molecular Biology 39: 312-328.
Wilson, M. Afin: Assembly finishing v.2016.09.17. 2016. https://github.com/afinit/afin.
Recibido: 24 de abril de 2024
Revisado: 13 de mayo de 2024
Corregido: 9 de agosto 2024
Aceptado: 10 de agosto 2024